Anything that has mass and takes up space.
What is matter?
This is the number of known elements in God's creation.
What is 118?
Name at least 3 of the 6 elements that are used in most organic molecules?
CHNOPS
Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, Sulphur
This is one of the most important covalently bonded compounds on Earth.
What is water?
Monosaccharides (monomers) are the building blocks of _____________.

What are carbohydrates?
Is a solution with a pH of 12 considered acidic or basic?
What is basic?
Scientists have performed detailed experiments that provide ample evidence for this unseeable building block of life.

What are atoms?
This is a chemical that results when two or more atoms join together chemically.
What is a molecule?
This is a chemical bond formed when one or more electrons are transferred from one atom to another.
An attractive force that holds molecules of the same substance together.
What is cohesion?
Your hair and fingernails are made up mostly of this.

What are proteins?
Enzymes do what for a chemical reaction?
Enzymes lessen the amount of activation energy needed in a chemical reaction
Subatomic particles of an atom.
What are protons, neutrons, and electrons?
This is a molecule that contains atoms of at least two different elements.
What is a compound?
This is a chemical bond formed by the sharing of electrons between two or more atoms.
What is a covalent bond?
This is a weak electrical attraction between a partially positive hydrogen atom and a partially negative atom of another molecule.
What is a hydrogen bond?
These are made up of fatty acids and all are hydrophobic which means they don't mix with water.

What are lipids?
What is the term for unraveling enzymes due to heat and pH changes?
How many total atoms are in a glucose molecule?
C6H12O6
24 total atoms
This is what happens if you breathe in a substantial amount of individual oxygen atoms.
What is die?
What happens to the charge of an atom when it loses an electron?
It becomes positively charged.
This is the substance in which other substances are dissolved.
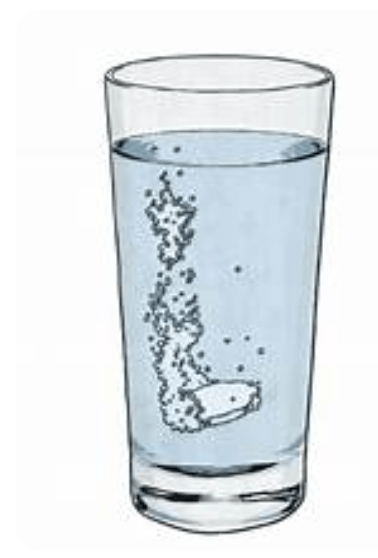
What is a solvent?
This helix coils around proteins to form chromosomes, which are the vehicles of inheritance. This structure houses genetic information for the organism.
What is DNA?
What holds the nucleotide bases together in the double helix of DNA?
hydrogen bonds
The vast majority of an atom's properties are determined by the number of these that it has.
What are electrons (or protons)?
Isotopes are one of several forms of an element, each containing the same number of protons but a different number of ______________.
What is neutrons?
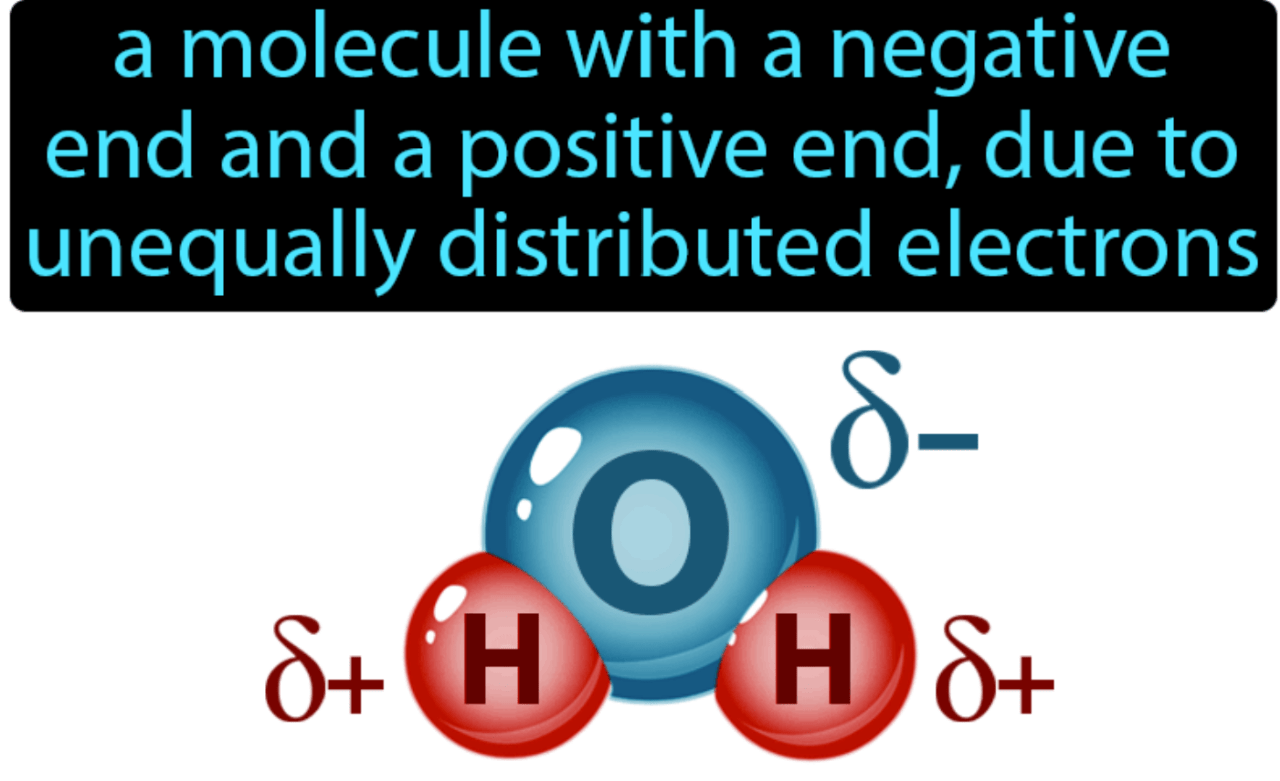
What is a polar compound?
Polar covalent molecules like sugar, dirt, and even gold can be broken down by water. We refer to it as a ______________ __________________.

What is universal solvent?
The building blocks of proteins are called________.
What are amino acids?
The 20 amino acids vary only in their _________ groups. Choose from the following:
carboxyl
side
amino
What is side group?