This is the solubility limit of sUA.
What is 6.8 mg/dL?
In the DISSOLVE protocol, these three disease state characteristics were used for the inclusion criteria that defined patients as having a "history of symptomatic gout".
What are...
1. > 3 gout flares within 18 months of screening,
2. presence of > 1 gout tophus and
3. current diagnosis of gouty arthritis
The mean baseline sUA level in both treatment groups of the pooled DISSOLVE data
What is 8.6 mg/dL?
True or False: Oral sirolimus is delivered to lymph nodes at the same level of concentration as IV NAS
False
This is the definition of a responder in the pegloticase pivotal trials
What is a patient with plasma UA less than 6.0 mg/dL for 80% of the time or longer during both months 3 and 6?
In humans, this is the organ system responsible for excreting approximately one-third of uric acid.
What is the GI Tract?
The 5 countries where DISSOLVE II was conducted
What is US, Russia, Ukraine, Serbia, and Georgia.
This is the Grade of the infusion reaction seen with SEL-110 only
What is Grade 2?
What is induction of antigen-specific regulatory T cells (or Tregs)?
The number of hours post infusion monitored for IRs in Krystexxa vs NASP
2 hours vs 1 hour
This cytokine is noted to have a pivotal role in the initiation of Gout flares
What is IL-1 Beta?
A patient would NOT have been excluded from the DISSOLVE studies if they had an allergy to the following medication:
i. Pegloticase
ii. Certolizumab pegol
iii. Pegfilgrastim
iv. Pembrolizumab
What is Pembrolizumab?
Name the advantages of NASP in CKD patients
what is.... improvement in eGFR, sUA reduction, with no additional safety concerns.
Pegadricase catalyzes the oxidation of uric acid to this, before it spontaneously degrades into allantoin and carbon dioxide.
5-hydroxyisourate
This is not a warning/precaution for pegloticase based on the prescribing information
A. Congestive heart failure
B. Anaphylaxis/Infusion reactions
C. Chronic kidney disease
D. G6PD Deficiency associated hemolysis and methemoglobinemia
What is C. Chronic kidney disease?
The percentage of the CRG population that is currently utilizing uricase therapy
What is ~ 2-3%?
This is the percentage of patients in the DISSOLVE II study that were from the US
What is 37%?
In DISSOLVE, there were 7 anaphylactic infusion reactions. This is the reason why we have reaction graphs for ONLY 6 of them.
What is.. because 1 patient never received their dose of pegadricase as their IR came from the sirolimus infusion.
Sirolimus is an acute inhibitor of this...
What is mTORC1?
mTORC2 can be inhibited with chronic treatment
These 4 immunosuppressants have data with Kystexxa
what are mtx, mycophenalate, leflunomide, azathrioprine?
Dysfunctional variants of this gene, involved with uric acid transport, are known to predispose someone to increased uric acid levels and chronic gout
What is ABCG2?
(other answers also acceptable)
This percentage of patients were tophaceous in the dissolve I and II trials (Two different %s)
What is....
56-57% dissolve I
66-67% dissolve II
Percentages apply to all groups, tx and placebo
In the pooled data, response rate was __% of the US HD cohort, and ____% of the ex-US HD cohort.
What is 55% and 45%
Walk us through the attached MOA
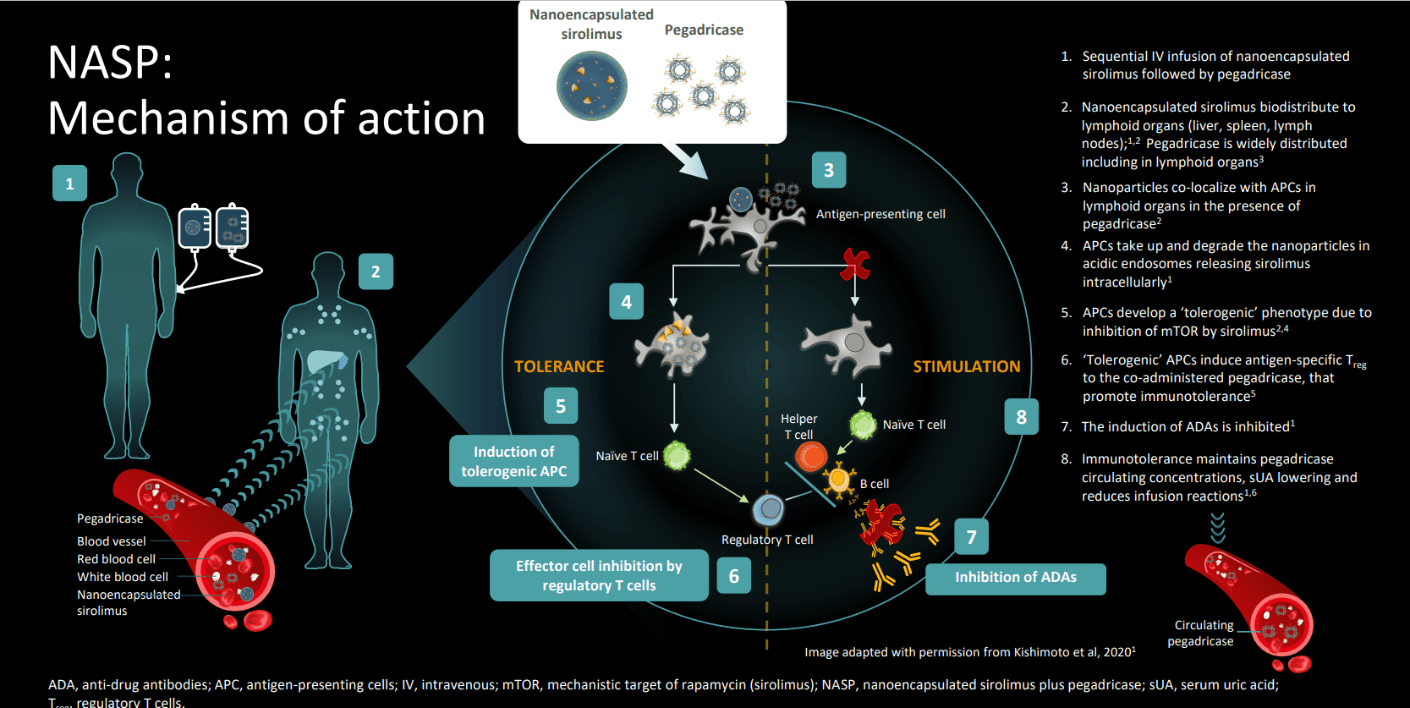
Must have all steps correct
Approximately this many times as many patients developed Anti-PEG antibodies in the PBO cohort compared to the MTX cohort in the MIRROR Trial.
What is 2 times?
These 3 things are the main drivers of metabolic dysfunction in patients with CRG
What are...
inflammatory pathways
endothelial dysfunction
oxidative stress
This is the antihistamine and steroid premedication schedule for NASP. (Must get entire regimen correct including dose and timing)
What is...
• Prednisone (40 mg) oral (PO) approximately 24 (± 12) hours prior to dosing
• Fexofenadine (180 mg) oral (PO) approximately 12 (± 2) hours prior to dosing
• Fexofenadine (180 mg) oral (PO) approximately 2 (± 1) hours prior to dosing
• Methylprednisolone (100 mg) (or equivalent) up to 125 mg, depending on patient weight, IV approximately 1 (± 0.5) hours prior to dosing
Walk through the NON-RESPONDER curve of DISSOLVE’s mean sUA over time, including the proportion of patients still receiving NASP at TP6 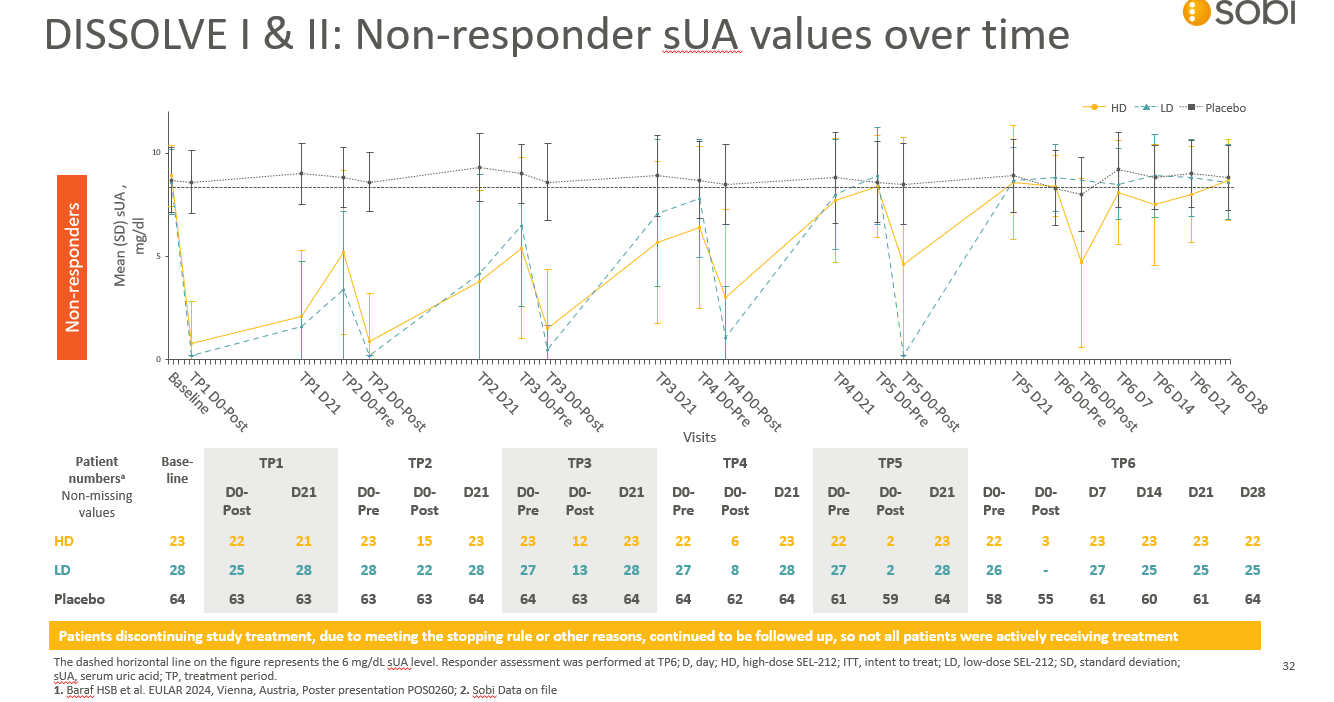
What is...
At TP6 still receiving NASP
HD - 3
LD – 0
How does the MOA of NAS differ than the MOA of methotrexate, and what makes NAS targeted?
Directors to judge
What you would say to a Rheumatologist when they ask you why they should use NASP vs Krystexxa
Directors to award points (directors cannot earn on this question)