(Must be in nuclear notation)
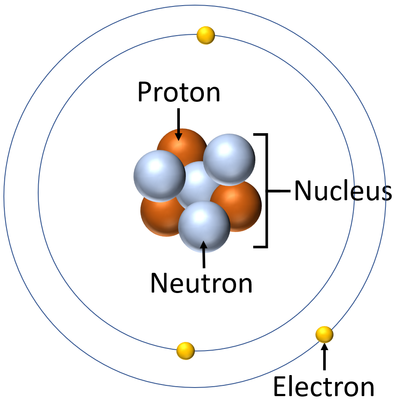
This particle has a positive charge.
A proton
The atomic number is the same as the number of this particle.
Proton or Electron
This element has 20 protons and 20 neutrons
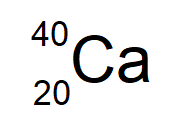
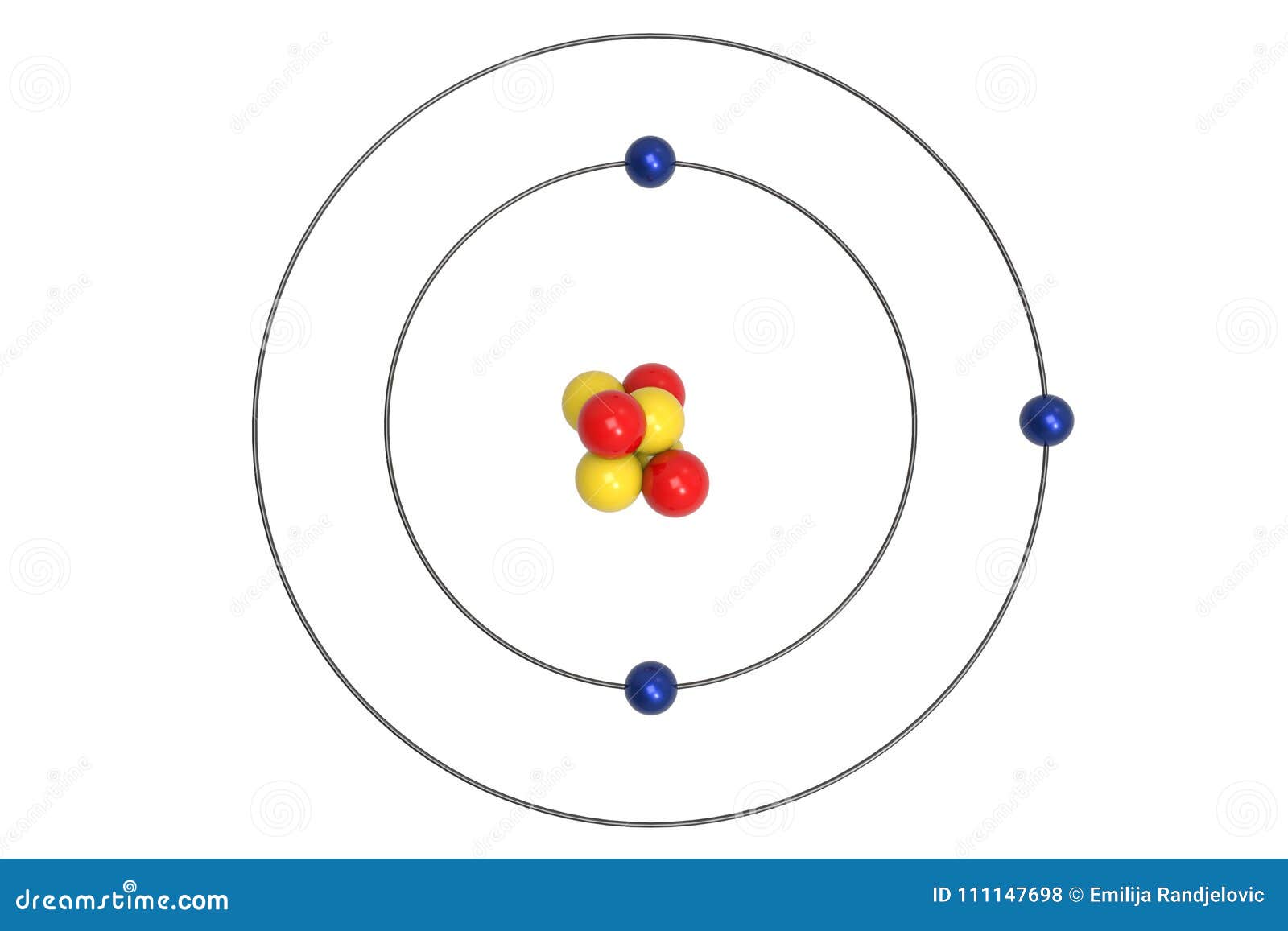
lithium-6
This particle's only contribution to the atom is to give the atom more mass.
A neutron
I show you the element, mass number and number of protons all in one. If you know your stuff - you can determine the number of neutrons too.
Nuclear symbol
Nitrogen with 8 neutrons
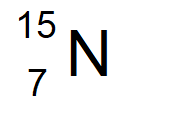
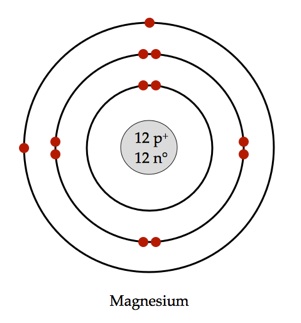
Magnesium-24
They are found in the nucleus.
protons and neutrons
When atoms have a different number of electrons and protons.
Ions
Atomic number 13 and a mass of 28


potassium-39
Have a neutral charge
Neutrons
I tell you the number of protons and electrons of an element
atomic number
6 Protons and 8 neutrons
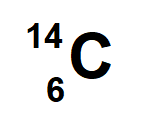
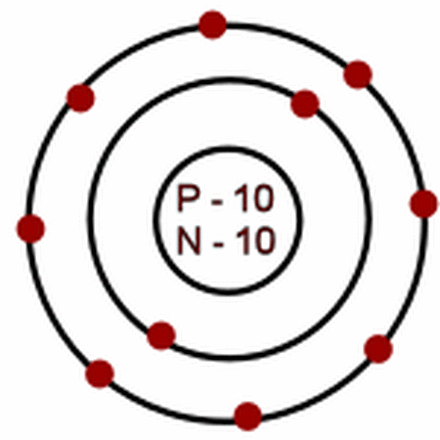
neon-20
This particle has virtually no mass.
Electrons
I am the addition of the number of neutrons and protons
atomic mass
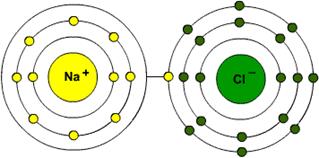
Sodium with a mass of 23 and 1 electron missing
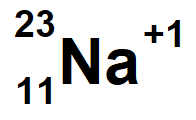
lanthanum-139
Draw a labeled atom (neutron, proton, electron, nucleus)
I am often represented by the letter X
chemical symbol
Chlorine with 19 neutrons and an extra electron
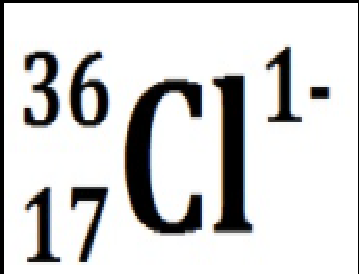
Draw the bohr model of sodium missing an electron AND chlorine with an extra electron