Pre-Test
Charges
Subatomic
Numbers
Heating Curve
100
This is the smallest part of an element.
What is an atom?
100
Objects with the same charge do this when they come close to each other.
What is repel?
100
Three subatomic particles.
What are protons, neutrons, and electrons?
100
The sum of protons and neutrons in the nucleus of an atom.
What is mass number?
100
The name given to this type of graph

What is HEAING CURVE?
200
Neutrons are located outside of the nucleus. True or False?
False!
200
Objects with different charges do this when they come close to each other.
What is attract?
200
Located outside of the nucleus and have a negative charge.
What are electrons?
200
The number of protons in an atom of a certain element.
What is atomic number?
200
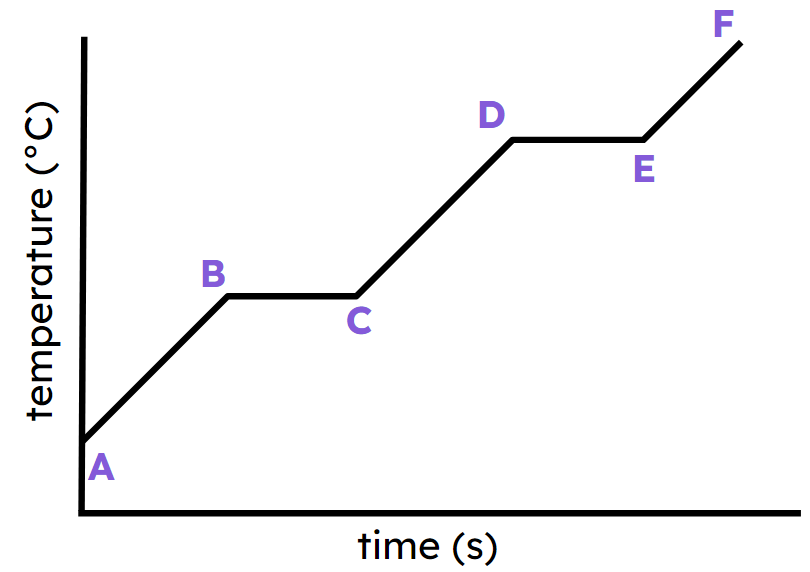 State ONE section of the graph where a change in temperature is happening?
State ONE section of the graph where a change in temperature is happening?
What is from A --> B, C-->D and E-->F?
300
Electrons are located outside of the nucleus. True or False?
True!
300
When an atom gains an electron it has this kind of electrical charge.
What is negative?
300
Located inside of the nucleus and have a positive charge.
What are protons?
300
The atomic number of fluorine.
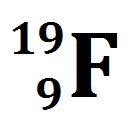
What is 9?
300
Which section of the graph has a phase change (or changing state) taking place?

What is B--> C, D --> E?
400
Protons and neutrons are about the same size. True or False?
True!
400
When an atom loses an electron it has this kind of electrical charge.
What is positive?
400
Atoms of different elements have different numbers of these.
What are protons?
400
The mass number of Potassium.
What is 39?
400
What is the melting point of the substance?
What is 70oC?
500
This is the smallest subatomic particle.
What is an electron?
500
An atom that is neither positively or negatively charged.
What is neutral?
500
An atom with the same number of protons but different number of neutrons.
What is an isotope?
500
Mass number - atomic number = ___________.
What is the number of neutrons?
500
How many minutes does it take for the substance to boil?

What is 5 minutes?