Pre-Test
Electrons
Subatomic
Numbers
Modern Theory
100
This is the smallest part of an element.
What is an atom?
100
Objects with the same charge do this when they come close to each other.
What is repel?
100
Three subatomic particles.
What are protons, neutrons, and electrons?
100
The sum of protons and neutrons in the nucleus of an atom.
What is mass number?
100
An atom is in this state when all of its electrons have the lowest possible energies.
What is a ground state?
200
Neutrons are located outside of the nucleus. True or False?
False!
200
An atom that is neither positively or negatively charged.
What is neutral?
200
Located outside of the nucleus and have a negative charge.
What are electrons?
200
The number of protons in an atom of a certain element.
What is atomic number?
200
This state is less stable than the ground state.
What is an excited state?
300
Element ___ has 20 protons, 22 neutrons, and 18 electrons
What is calcium?
300
When an atom gains an electron it has this kind of electrical charge.
What is negative?
300
Located inside of the nucleus and have a positive charge.
What are protons?
300
The atomic number of Oxygen.
What is 8?
300
Electrons move from one of these to another when atoms gain or lose energy.
What is an energy level?
400

Which element is the unknown element based on the emission spectra?
What is Strontium?
400
The electron configuration of this element is 1s22s22p63s1
What is sodium (Na)?
400
Atoms of different elements have different numbers of these.
What are protons?
400
The mass number of Potassium.
What is 39.098?
400
The region of space around the nucleus where an electron is likely to be found.
What is an orbital?
500
This is the smallest subatomic particle.
What is an electron?
500
Iron is an element that we use on a daily basis and its what binds oxygen in our blood which allows us to breathe. Write the electron configuration for iron (Fe).
What is 1s22s22p63s23p64s23d6 or [Ar]4s23d6?
500
An atom with the same number of protons but different number of neutrons.
What is an isotope?
500
Mass number - atomic number = ______ __ ________.
What is the number of neutrons?
500
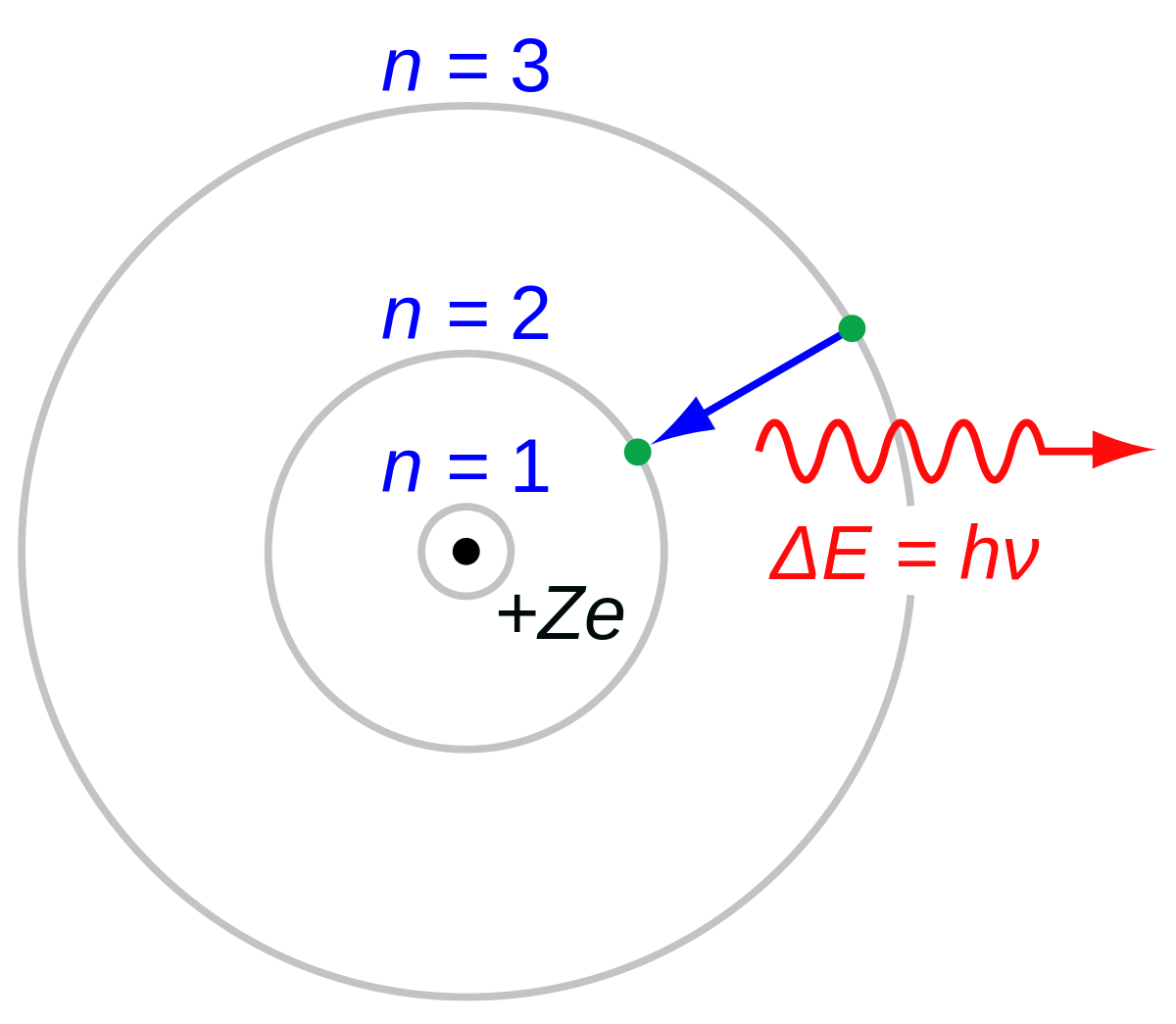 Scientists use the Bohr model to show this process which provides us with light.
Scientists use the Bohr model to show this process which provides us with light.
What is atomic emission?