Atomic Mass
The Electron Cloud
This subatomic particle has a negative charge
Electron
________are atoms of an element with different numbers of neutrons.
isotope
Draw the complete nuclear symbol for an alpha particle

What does "c" symbolize? What number is associated with it?
Speed of Light
3.00 x 108 m/s
Who created the first model of the atom?
John Dalton
The average mass of all forms of the atoms of an element based on the abundance of that element is called .
average atomic mass
nuclear reaction that breaks a parent nucleus into smaller nuclei
fission
What does the letter symbolize in electron configuration?
What are the letters that we use?
sublevel
s,p,d,f
Which subatomic particle affects the mass number, charge, and identity of the atom.
Protons
Protons plus neutrons equals
mass number
What type of nuclear reaction is shown below?
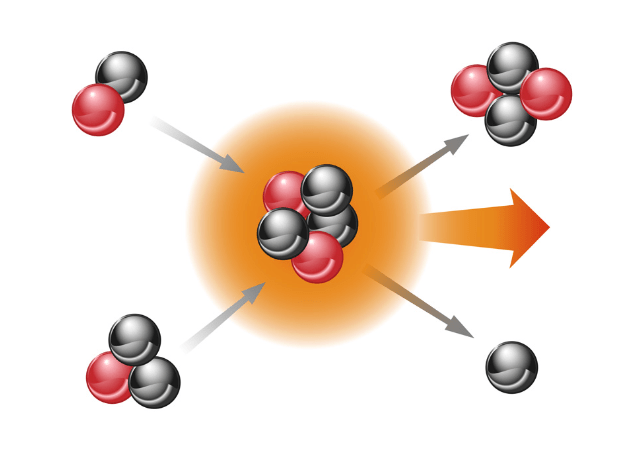
Fusion
Write the electron configuration for Carbon
1s22s22p2
Label all the models by who created them in the order of the picture. 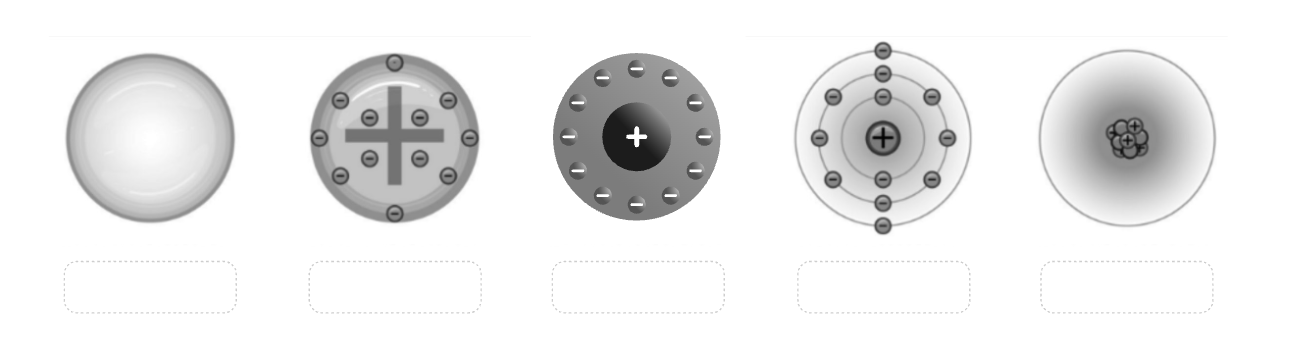
Dalton, Thomson, Rutherford, Bohr, Heisenberg.
Oxygen has three isotopes: oxygen-16, oxygen-17, and oxygen-18. The atomic mass reported on the periodic table is 16.00.
Which isotope of oxygen is the most abundant?
Oxygen-16
radiation released in a nuclear reaction that results in the release of energy yet leaves both the mass and the identity of the parent nucleus unchanged
gamma ray
What is the Noble gas configuration for Iodine
[Kr]5s24d105p5

4, 2, 3, 5, 6, 7, 1
Thallium has two stable isotopes: Tl-203 and Tl-205.
Their relative abundances are 29.5% and 70.5%, respectively.
What is the average atomic mass of thallium?
204.41 amu
Balance the following equation and determine if this is a fission or fusion reaction.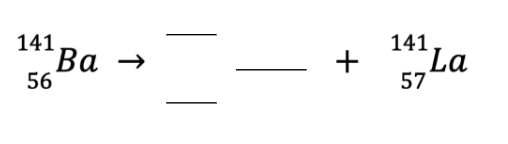
0-1 e
Fission
One of the photons of light from the aluminum emission spectrum has a wavelength of 4.66 x 10−7 m.
Where on the electromagnetic spectrum does this fall? What is its frequency? What is its energy?
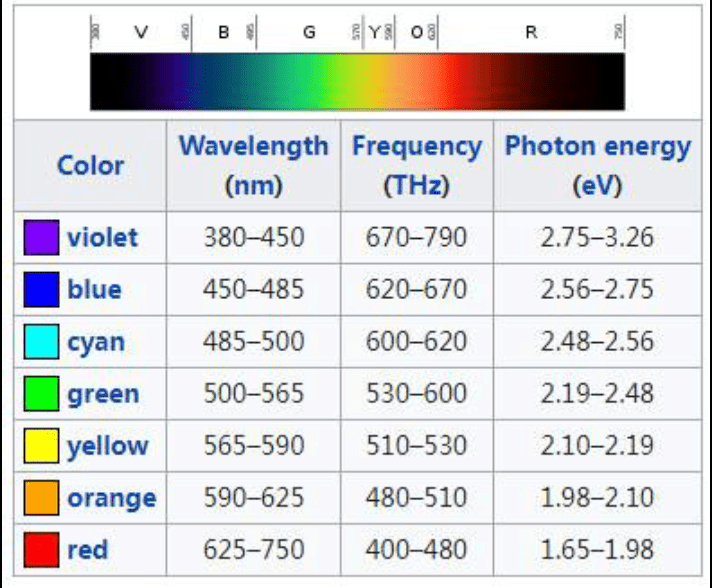
Blue
6.43 x 1014 Hz
4.26 x 10-19 J