What are the Alkali Earth Metals?
The atomic number is the same as the number of this particle.
What is a proton?
The isotope symbol of Calcium-40, an element with 20 protons and 20 neutrons.
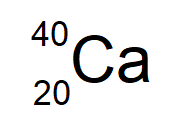
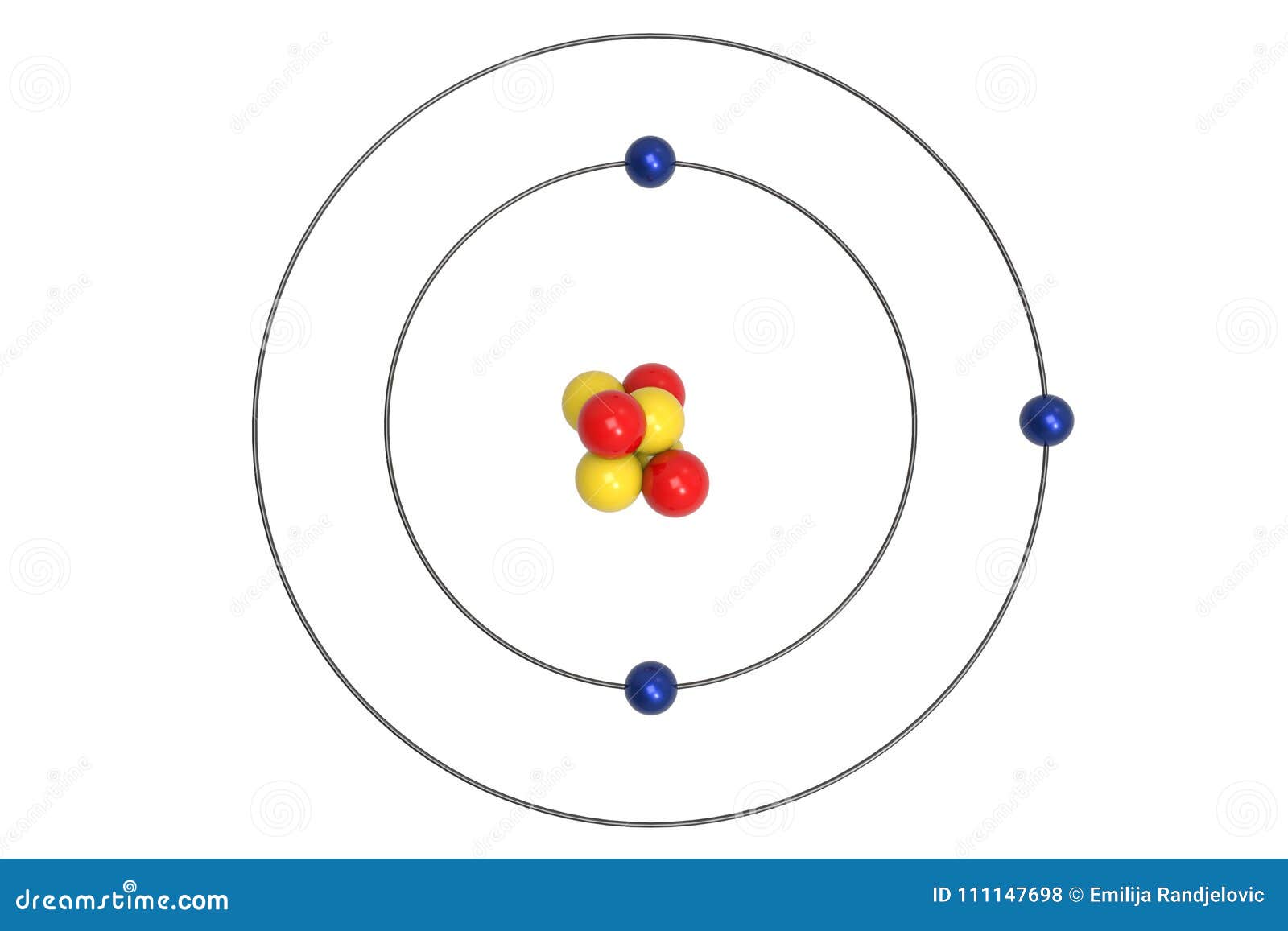
lithium-6
A nuclear reaction involves ONLY this part of the atom.
What is the nucleus?
Flammability is an example of a _________________
chemical property
This group is known as the halogens and always forms -1 ions
What is Group 17?
This is a group of atoms that all share the same number of protons but have different numbers of neutrons.
What is an isotope?
The nitrogen isotope with 8 neutrons
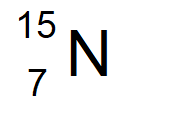
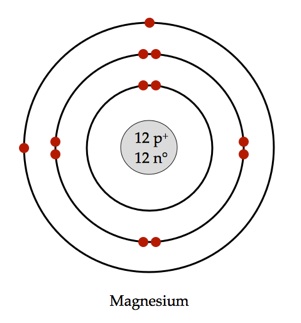
Magnesium-24
This nuclear reaction is defined as an atomic nucleus splitting into smaller pieces.
What is fission?
Freezing point is an example of a ________________
physical property
This group's atoms all have 5 valence electrons and form -3 ions.
What is Group 15/the Pnictogens?
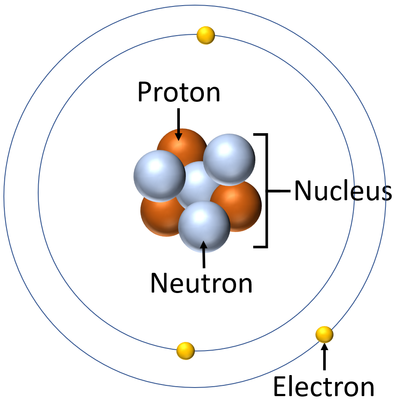
Found in the nucleus.
What are protons and neutrons
When atoms have a different number of electrons and protons.
What are Ions
Atomic number 13 and a mass of 28


potassium-39
The length of time it will take half of a radioactive isotope to go through decay.
What is half-life?
A cube has a density of 10g/cm3, you cut it in half, what is the density now?
10g/cm3
This element has 6 valence electrons in its outer shell and 3 total electron shells.
Sulfur
Have a neutral charge
What are neutrons?
A negative ion
anion
6 Protons and 8 neutrons
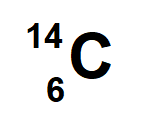
neon-20
An alpha particle is also known as a nucleus of this atom, since it is made up of 2 protons and 2 neutrons.
What is helium?
This is a property of matter that is independent of the amount of substance.
What is an intrinsic property?
The most common isotope of Gold contains these subatomic particles in these numbers:
Protons - 79
Neutrons - 118
Electrons - 79
positive ion
cation
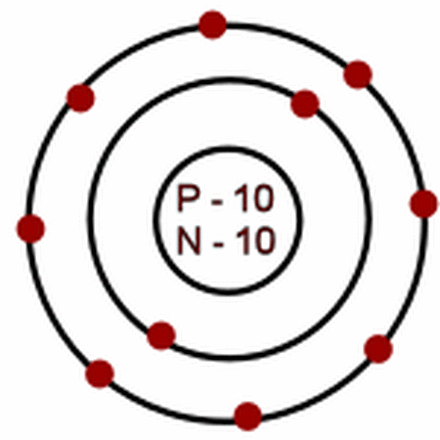
Sodium with a mass of 23 and 10 electrons
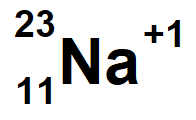
lanthanum-139
This type of nuclear radiation penetrates more shielding than any other, and is used to start nuclear chain reactions.
What is neutron radiation?
A cylinder with this density might have a volume of 9.0 mL and a mass of 24.3g.
What is 2.7 g/mL?
All elements' nuclei are unstable (and therefore radioactive) after this heavy metal.
What is Lead (Pb, Element 82)
Draw the Bohr model for Lithium-7 labeled with: neutron, proton, electron, nucleus
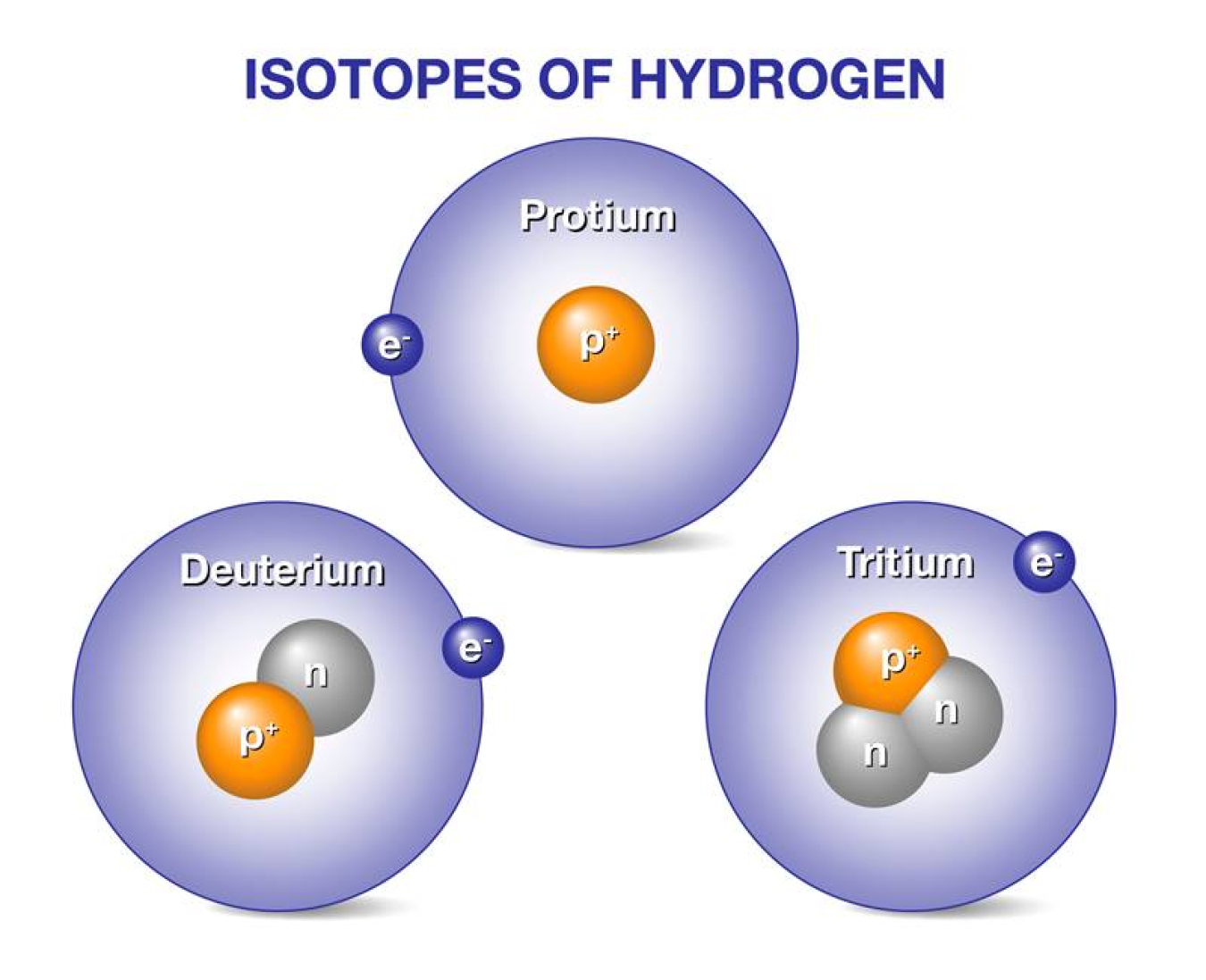
Draw and name the 3 isotopes of Hydrogen
Chlorine with 19 neutrons and 18 electrons
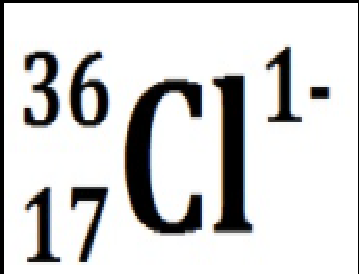
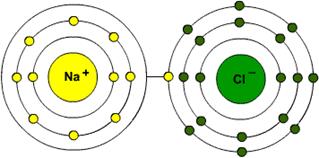
Draw the bohr model of sodium missing an electron AND chlorine with an extra electron
Radon-222 emits an alpha particle with 2 neutrons and 2 protons. What is the new atom & isotope formed after the alpha emission? (Give an element name and its mass number)
Polonium-218
A cube has a mass of 50g and has sides that measure 5cm each. What is the density? Will it sink or float?
0.4 g/cm3 it will FLOAT