A part of the atom with a positive charge
What are protons
The electron configuration for Helium
What is 1s2
The atomic number of the element Calcium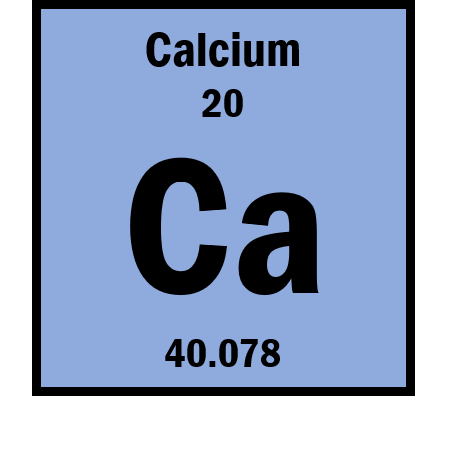
What is 20?
Hund's rule means this
What is every orbital must be filled with one electron before any other orbital is occupied twice
The Bohr model represents this element

What is Hydrogen
This is what electrons revolve around
What is the nucleus
The shorthand configuration for the element Calcium
What is (Ar) 4s2
To find the number of neutrons the atomic number is subtracted from this number
what is mass number
The Bohr model represents this element
What is Argon
True or False
As a wavelength gets shorter, the energy decreases
What is False
According to this theory, electrons circle the nucleus in a specific orbital going in a certain direction
What is the wave mechanical model
True or False
A D orbital can hold up to 12 electrons
What is False
The number of protons and neutrons in the oxygen isotope 18

What is 8 protons and 10 neutrons
This principle states electrons must have opposite spins and each orbital can hold a maximum of two electrons
What is the Pauli exclusion principle
On the EM spectrum these waves are the longest
What are radio waves
In this type of decay two protons are lost, while in this other type of decay there is no change in protons
What is alpha and gamma
Long hand electron configuration of the element Chlorine
What is 1s2 2s2 2p6 3s2 3p5
The number of protons, neutrons, electrons and the mass number of 40Ca+2
What is 20 protons and neutrons, 18 electrons and 40 is the mass number
Draw the orbital diagram of Chlorine
In the image below the transition of the electron emits this part of the EM spectrum

What is visible light
Each part on the image below represents

What is 1 is the atomic number, H is the atomic symbol, Hydrogen is the element name, and 1.008 is the atomic mass
True or False
An isotope's electron configuration will be different from the neutral atom's electron configuration because of a change in the electrons of the isotope
What is False
DOUBLE JEOPARDY!!!!
If correct add another 500 points
Write the isotope symbol for an element with 17 protons, 18 neutrons and electrons, and a mass number of 35
What is 35Cl-1
The number of electrons in the last P orbital of Nitrogen
What is 3
RISKY MOVES!!
This question is risky to answer wrong!!!!!
Choice of keep or pass to another team
True or False
In the visible light spectrum shorter wavelengths are referred to as bluer
What is True
If correct add another 500 points, but if incorrect take away an additional 500!!!
