How many valence electrons does Chlorine have?
7 valence electrons
What are the diagrams called in the image?
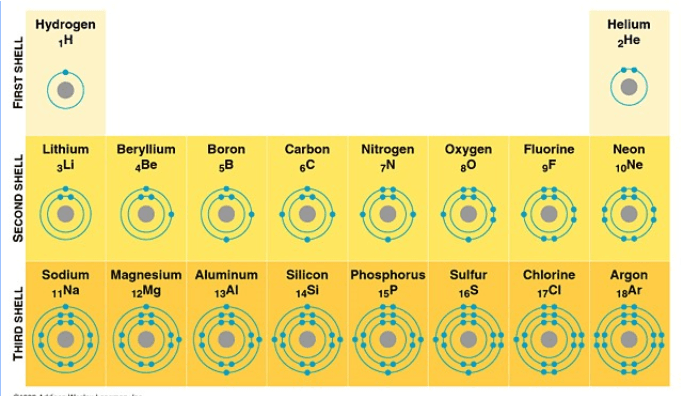
Bohr Models.
Which subatomic particle is neutral or has no charge?
Neutron
Which element(s) has reactivity that is similar to Iodine (I)?
A. Xenon (Xe)
B. Bromine (Br)
C. Fluorine (F)
B. Bromine (Br) and C. Fluorine (F)
What is the density formula?
density=mass/volume
What are the columns on a periodic table called? What do the columns determine about an element?
Groups. They determine the number of valence electrons an element has.
What is the maximum number of electrons the 2nd orbital/energy level/electron cloud, can hold?
8 Electrons.
Which subatomic particle has a positive charge?
Proton
How many elements are on the Periodic Table of Elements?
118 Elements
Elements in the same group share what property?
The same number of valence electrons.
What number do we use to identify an element on the Periodic Table?
The Atomic Number or the Number of Protons
What are found on the outermost energy level of an atom?
Valence electrons
Where are protons and neutrons located in an atom?
Inside the Nucleus
How many Protons does Arsenic (As) have?
Arsenic (As) is Atomic Number 33, therefore it has 33 Protons.
Which Group on the Periodic Table are considered "Noble Gases"?
Group 8A or 18.
What are the rows on the periodic table called? What property do they tell you about an element?
Periods. They tell you the number of energy levels an element has.
Name the 3 subatomic particles of an atom.
Protons, neutrons, and electrons.
Which subatomic particle has a negative charge?
An Electron
What is the formula for finding the mass number of an element?
Mass number= Protons + Neutrons
A student measures the mass and volume of a small cube made of an unknown metal. The mass of the cube is 25.0 g, and the volume of the cube is 3.19 cm3. What is the density?
7.84 g/cm3
Which TWO Groups of elements are considered "very reactive"?
Group 1A and Group 7A. Elements with 1 or 7 valence electrons are very reactive.
Which element is the least reactive, Neon or Aluminum?
Neon because it has 8 valence electrons or a full outer energy level of electrons.
What subatomic particle is located outside the nucleus, in the electron cloud/energy levels?
Electrons
What is the total number of protons, neutrons, and electrons in a neutral atom of Aluminum with an atomic mass of 28?
A=13
P=13
E=13
M=28
A=13
N=15
13+13+15=41
True or False: Density is a Physical Property?
True, Density is a Physical Property.