Postive subatomic particle
Protons
How many electrons can the first energy shell hold?
2
If you are shown a picture of a bohr model, what can you count to figure out which element it is?
electrons (or protons)
matter
What is the chemical symbol for Hydrogen?
H
Smallest part of an element that still has the same properties of that element-
Atom
Negative subatomic particles
electrons
What is the center of an atom called?
A student does an experiment testing the reaction between vinegar and baking soda.
Hypothesis-
If I add more baking soda, then the chemical reaction will be larger because the vinegar will have more substance to react.
What is the independent variable?
the amount of baking soda
Neon has an atomic number of 10. 10 neutrons and 10 electrons. What is the atomic mass of neon?
20
What is the chemical symbol for helium?
He
How many neutrons does copper have?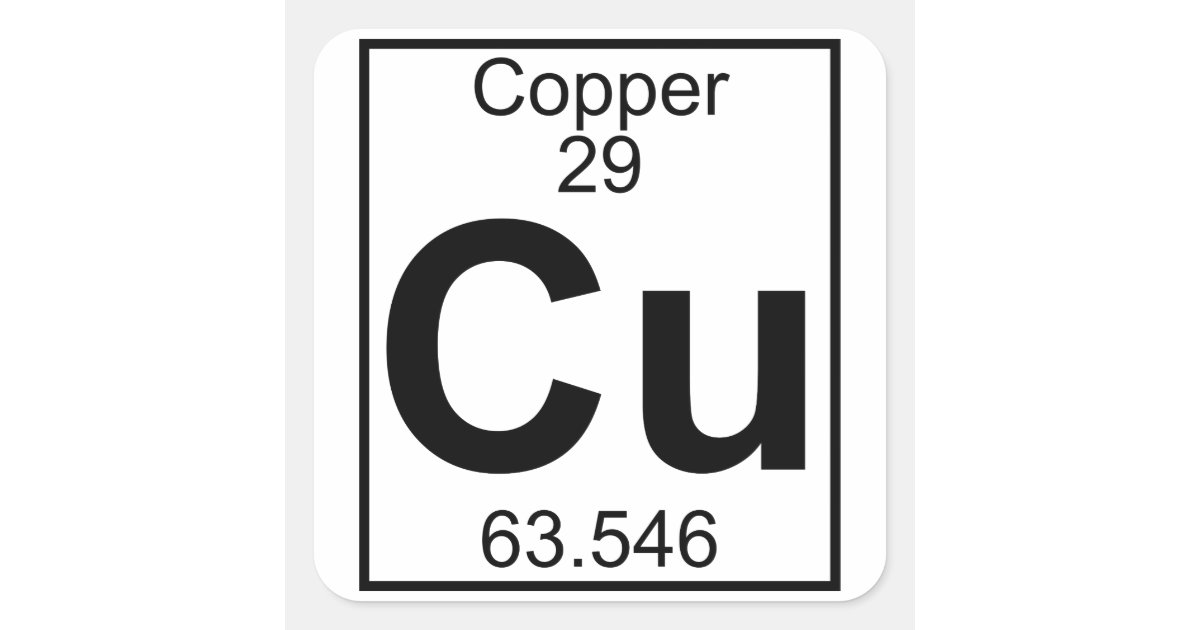
35
Neutral subatomic particles
neutrons
How many protons are shown in the below model of carbon?
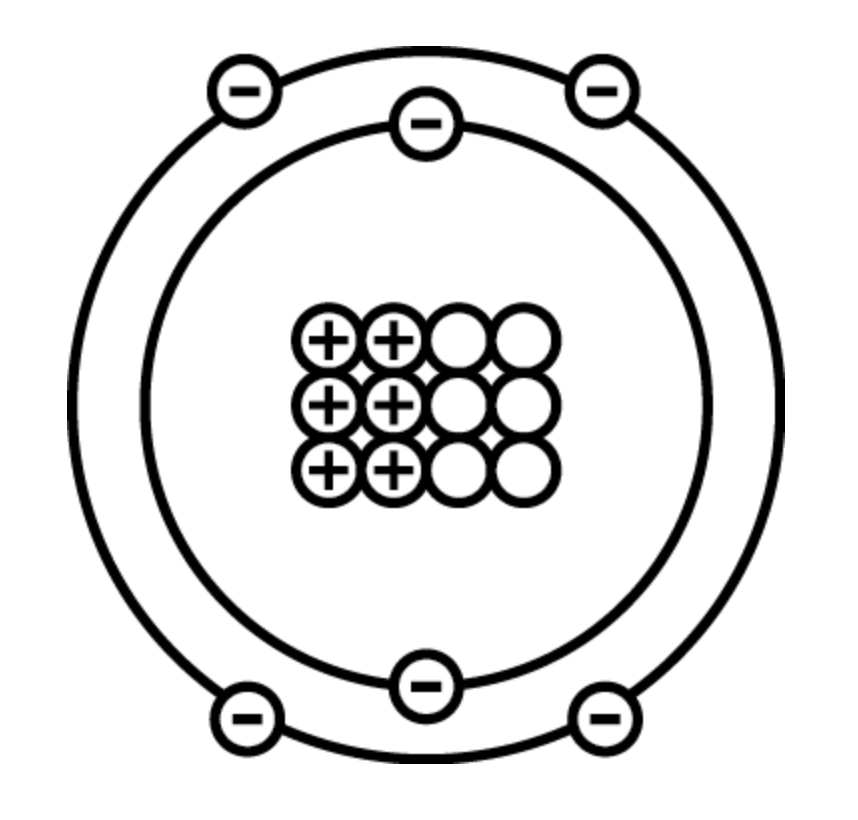
6
Most common element is Earth's atmosphere?
Nitrogen
When two elements bond together, they form a___________.
Compound
What is the chemical symbol for lithium?
Li
What is the atomic weight of calcium?
40.078
Where are the electrons located?
Outside the atom on the energy shells
The basic building block of all matter is-
atom
How many protons would an atom of iron have?

26
What is NOT true about elements?
A: Elements are chemically combined
B: Elements cannot be broken down
C: Elements are represented by a chemical symbol
D: Elements are only made of one kind of atoms
A: Elements are chemically combined
What is the chemical symbol for Boron?
B
Most abundant gas in the atmosphere?
Nitrogen
What subatomic particles are located in the nucleus?
Protons & neutrons
The atomic mass tells the number of __________ and ________ in the nucleus of an atom?
Most common element in Earth's crust and 2nd most common in atmosphere?
Oxygen
Which element square is below?
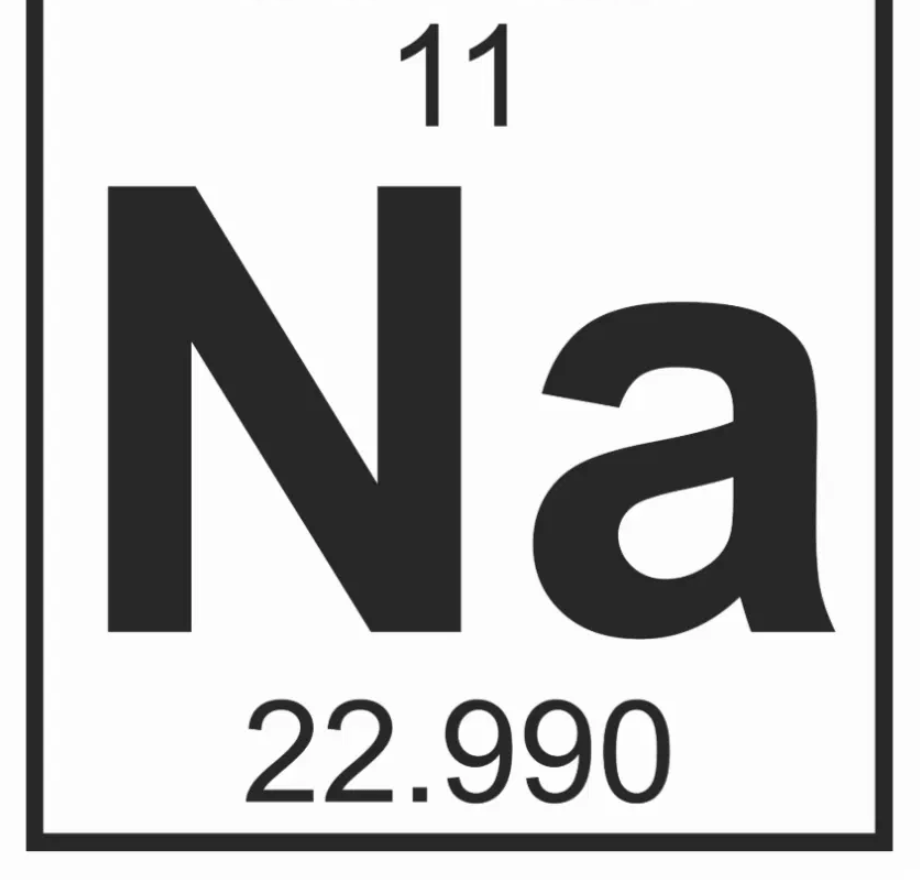
Sodium
What is the chemical symbol for Beryllium?
Be
Which 2 elements are in the below compound?
H2O2
Hydrogen and Oxygen
Which particles can be lost, shared, or gained from an atom when a chemical bond forms?
Electrons
Which element does the bohr model below represent?
Oxygen
How many atoms of iron are in the below molecule?
Fe2O3
2
Which example is a compound?
A: BO
B: Be
C: N
D: Fe
BO
What is the chemical symbol for silver?
Ag
What are the 3 major elements in Earth's Crust?
Aluminum, Silicon, and Oxygen