You might think it's a negative, but I don't mind being the smallest subatomic particle.
What is an Electron?
Pb, or not Pb - this element's symbol originates from Plumbum, its Latin name.
What is Lead?
Ooo, shiny! Most elements in the periodic table are what type of material.
What is Metal?
Are you sure? Are you certain? Is the evidence indubitable? Protons have this type of charge.
What is Positive?

Named after the genus of bumblebees, this feathered companion has now lived with Mr. Garlick for over 9 years.
Who is Bombus?
In the nucleus we're smashed nice and tight, there's 2 pieces that make this question right.
What are Protons and Neutrons?
While separate, sodium and chlorine are quite dangerous. When combined, they make table salt. This is the number of protons that separate them.
What are 6?
Elements in the same column or "group" in the periodic table have similar properties because they have the same number of these. If you're thinking electrons, you're not quite close enough.
What are Valence Electrons?
While not quite as explosive as their similarly named cousins, this group of metals is the 2nd most reactive.
What are the Alkaline Earth Metals?
Let me give you a hand in figuring out this state where Mr. Garlick was born and raised.
What is Michigan?
Switzerland and an atom with the same number of protons and electrons have this in common.
What is Neutral?
The number of neutrons in an atom of Oxygen-18.
What are 10?
We say these interesting elements are along the staircase of the periodic table - they're neither metals nor non-metals, but have properties of both.
What are Metalloids?
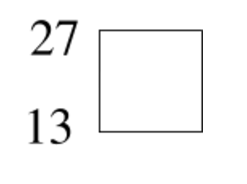
Fill in the blank - this elemental symbol completes the nuclide.

This car, with a model name that translates to "The Road," was Mr. Garlick's first - he still somewhat misses it to this day.
What is an El Camino (1986)?
Electrons in the outermost orbital of an atom are called this - be careful, they might want to react with you!
What are Valence Electrons?
Besides one being radioactive, this is the difference between an atom of Carbon-12 and an atom of Carbon-14.
What are 2 Neutrons?
Watch out, prop-in-science-video bathtubs - these are the most reactive type of metals, and can really heat things up when they lose their valence electrons.
What are Alkali Metals?
This group of elements has all the valence electrons they need - they don't want to react with anything.
What are the Noble Gases?
The number of years Mr. Garlick has spent in some college or another.
What is ten?
Our philosopher's stone is broken, and takes our gold and removes a proton from it. This is the element it gets turned into.
What is Platinum?
Write the nuclide symbol for this atom: 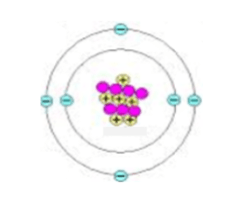
What is 
This letter represents the position of the most reactive Halogen.
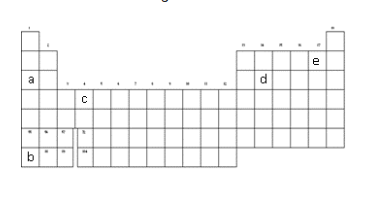
What is "e" ?
This letter represents the most reactive alkali metal. The poor British science guys weren't allowed to have any.
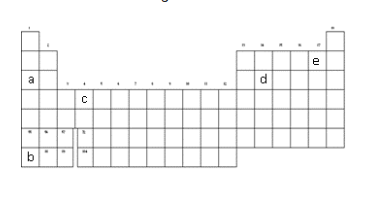
What is "b"?
You might NOT "remember," as the chain filed for bankruptcy in 2022 - this is the mall store where Mr. Garlick got his first job as a custom engraver.
What is Things Remembered?