This is the smallest part of an element.
What is an atom?
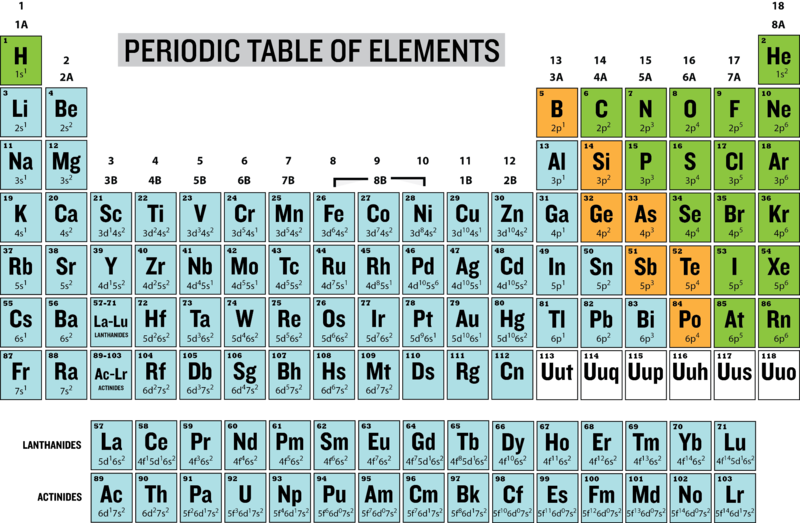
What are groups
The sum of protons and neutrons in the nucleus of an atom.
What is Atomic mass?
Name that character
Who is Bill Nye the Science Guy
Neutrons are located outside of the nucleus. True or False?
False!
This element is located in Group 1 Period 1
What is Hydrogen
This tells the number of protons in an atom of a certain element.
What is atomic number?
Name Mrs. Parham's favorite fast food chain
What is Chic-fil-a
Electrons are located outside of the nucleus. True or False?
True!
Using your periodic table name the element located in Period 4, Group 10
What is Nickel
Located inside of the nucleus and have a positive charge.
What are protons?
The atomic number of Argon.
What is 18?
 Name the Artist and name of their solo album
Name the Artist and name of their solo album
What is Quavo, Huncho
Protons and neutrons are about the same size. True or False?
True!
This group holds the noble gases neon, argon, krypton, xenon, and radon.
What is Group 18
Atoms of different elements have different numbers of these.
What are protons?
The mass number of Potassium.
What is 39.098?
Name that dance....
What is the "running man"
This is the smallest subatomic particle.
What is an electron?
Elements in the same group/ column have ____ properties
What is similar
The atomic number tells us the _____ of an element
What is the identity
Mass number - atomic number = ______ __ ________.
What is the numer of neutrons?
What is Mrs. Parham's son going to be for halloween?
What is a dinosaur?