What is the charge of a proton?
positive
What is the charge on a neutron?
Neutral means no charge.
What is the charge on the electron?
negative
What is the basic building block of matter?
atom
Who was the first person to describe the atom?
Democritus
Where is a proton located in the atom?
Nucleus
Where are neutrons located in the atom?
Nucleus
Where are electrons located in the atom?
electron cloud
What do we call a material made up of only the same atoms? (hint: all gold atoms)
an element
Using a periodic table, what element has the atomic number 2?
Helium
What is the weight of a proton?
1 amu
What is the weight of a proton?
1 amu
What is the weight of an electron?
1/2000 amu
If an atom has 3 protons, 3 neutrons, and 3 electrons, the atom is said to be...
neutral
Using the periodic table, what element has 10 protons?
Neon
How many protons are in the oxygen atom?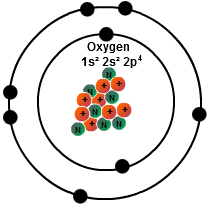
8 protons
True or False: The number of neutrons in an atom can make it stable or unstable.
True
How many electrons can fit in the first energy level of an atom?
two
If an atom has 7 protons, 7 neutrons, and 10 electrons, the atom is said to be...
an ion (-3 charge)
Using the periodic table, which element has the least number of protons?
Hydrogen
Why is the number of protons important in an atom?
The number of protons are unique to each different atom. They give the atom it's name.
How many neutrons are represented in this Bohr model of the Oxygen atom?
8 neutrons
 How many electrons are in the second energy level of this Bohr model?
How many electrons are in the second energy level of this Bohr model?
six
If an atom has 3 protons, 4 neutrons, and 3 electrons, what is the atom's atomic mass?
3 protons + 4 neutrons = 7
How many elements are listed on the Periodic Table?
118