The dense center of an atom.
What is nucleus
What is the Noble Gas Notation
Shortened electron configuration using a noble gas
Where are each of the subatomic particles located?
What is Protons and neutrons inside the nucleus and electrons outside the nucleus in the electron cloud.
How many electrons can fit inside the 2nd level?
What is 8 electrons? *BONUS* What happens to the energy of the electrons as they move from inner levels to outer levels?
What is the number of neutrons for 2713Al?
What is 14 neutrons? (27 = 13p+ + #n0)
The area which an electron is likely to be found.
What is electron cloud?
What is Hunds rule?
Electrons will spread out in a p, d and f orbital besides doubling up.
How are the 2 isotopes of Aluminum-26 and Aluminum-27 different.
What is they each have 26 p+ and 26 e-, their # of n are different *BONUS* If they differ by the # of neutrons, how does the mass of each compare?
An orbital that is sphere shaped and has one orientation.
What is S orbital
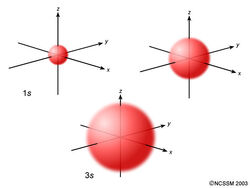
What is pauli exclusion principle?
All electrons have opposite spin (ml ) aka dont have the same e- configurations
Which subatomic particle gives an element it's unique properties (its identity)?
What is Protons *BONUS* WHY?
Draw the Bohr model for Aluminum

Which isotope of Aluminum is the most abundant Aluminum-26 or Aluminum-27?
What is Aluminum-27 *BONUS* WHY?
An orbital that is dumbell shaped and has 3 different orrientations.
What is p orbital

Tool used to measure wavelength or different colors of light.
What spectroscope?
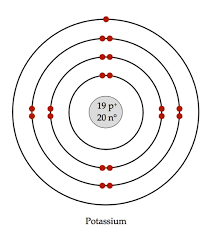
Draw the Bohr Model for Potassium
Ultimately the periodic table is separated into 4 different blocks based upon orbitals, what the blocks?
s, p, d and f
/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)
Why is color and/or light produced when an atom is excited?
What is electrons getting excited, moving from inner energy levels to outer levels, then back in