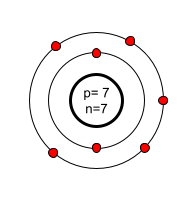
Where in the atom are the protons found?
Daily Double
Nucleus
Why do atoms have the same number of protons and electrons?
Protons are positive, electrons are negative
Makes them neutrally charged
I have 19 protons. Which element am I?
Potassium
These metals are found in group 2.
Alkaline-Earth Metals
On the periodic table, what is a “period”?
A row across.
This subatomic particle is so small that it does not factor into atomic mass.
Electron.
How many elements are there?
118
I have 83 electrons. Which element am I?
+100pt if you can explain how you know.
Bismuth.
The periodic table is organized so that the elements are grouped up based on their ______________.
Properties
Is this an element symbol? If not, explain. If it is, which element is it?
Mg
Yes, Magnesium
Electron cloud
I have 29 protons and 34 neutrons. Which element am I and what is my mass?
Daily Double
Copper, 63amu
My atomic number is 50. Which element am I?
Tin
This family of elements shares properties with both metals and nonmetals.
Metalloids.
Is this an element symbol? If not, explain. If it is, which element is it?
"HE"
No, both letters cannot be capitalized. HE is nothing, He is Helium.
How many protons does an atom of Strontium have?
38
I have a mass of 75 amu and 42 neutrons. Which element am I?
Arsenic
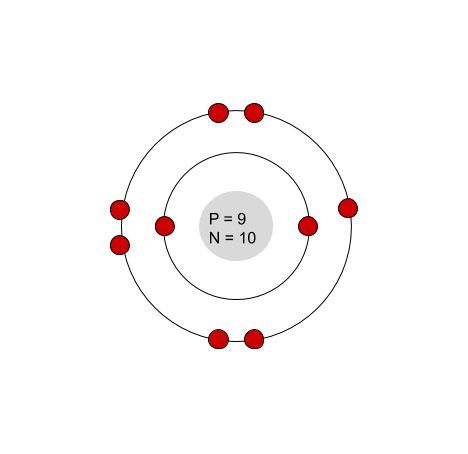 Which element is this?
Which element is this?
Fluorine
Which elements are considered Halogens?
Fluorine, Chlorine, Bromine, Iodine, Astatine
Fill in the information that is missing below: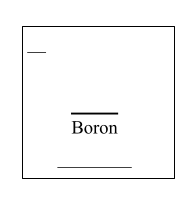
Atomic #: 5
Symbol: B
Mass: 10.81
I am a nonmetal, my mass is 80, and I can be found in the 4th period. Who am I
Bromine.
Explain why electrons do not calculate into the mass of an atom?
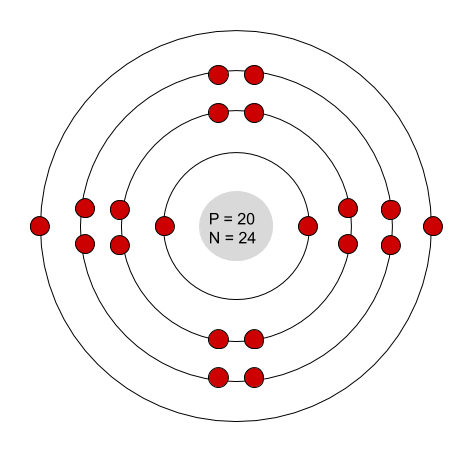 Which element is this?
Which element is this?
Calcium
This family of metals is extremely reactive with other elements.
Alkali Metals
Using the periodic table as a resource, draw a Bohr model for Nitrogen.