What do you call the inside of the atom?
nucleus
Which particle has a positive charge?
protons
What do you call this image?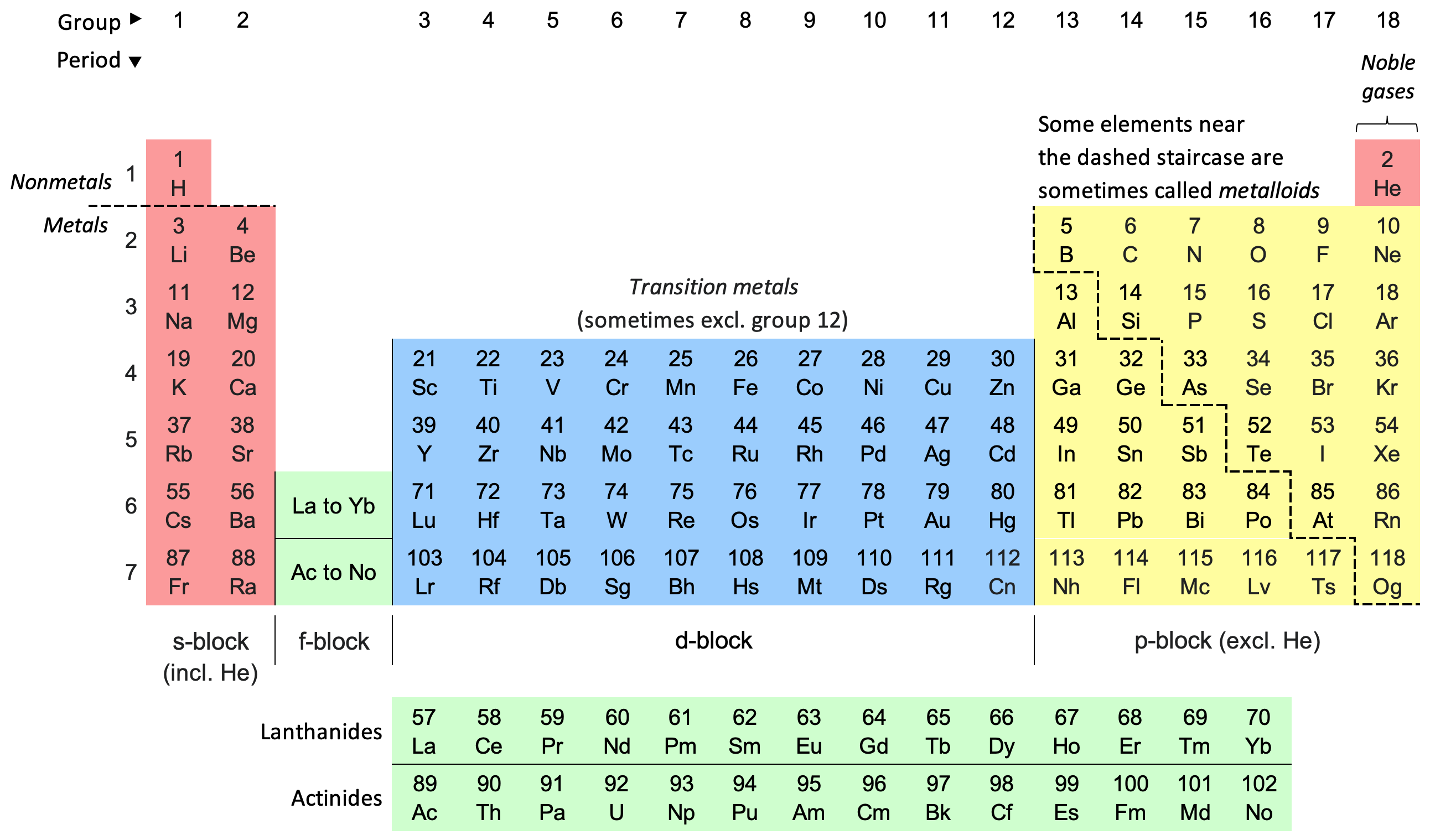
periodic table
The particles that makeup everything, living and nonliving, is called _____
atoms
Lithium has an atomic number of 2. How many protons does lithium ave?
2 protons
Where are the protons found in the atom?
In the nucleus
Which particle has no charge?
neutrons
What is the atomic number of this element?
2
What does the "APE" in A P E M A N mean?
atomic number = protons = electrons
Sodium (Na) has an atomic number of 11. How many electrons does Sodium have?
11 electrons
Where are neutrons located in the atom?
In the nucleus.
Which particle has a negative charge?
electrons
What 4 things did Greek philosophers think everything was made of?
Earth, air, water, fire
What does the M A N in A P E. M A N. mean?
mass number - atomic number = neutrons
How many protons, neutrons, and electrons does the atom have?

Protons = 6
Neutrons = 6
Electrons = 6
Where are the electrons located in the atom?
on the outside
Particles of different charge will ______
attract (move toward) each other.
What element accounts for 90 percent of the universe?
Hydrogen
What is an element’s atomic number?
The number of protons and the number of electrons in an atom
Titanium (Ti) has a mass number of 48 and an atomic number of 22, how many neutrons does Titanium (Ti) have?
26 neutrons.
What is an isotope?
atoms of the same element that have a different number of neutrons.
Particles of the same charge will _____
repel (push away) from each other
What 4 elements are living things made of?
oxygen, carbon, hydrogen, and nitrogen.
What is an element’s atomic mass?
The total number of protons and neutrons in an atom’s nucleus.
How many protons, neutrons, and electrons does Magnesium(Mg) have?
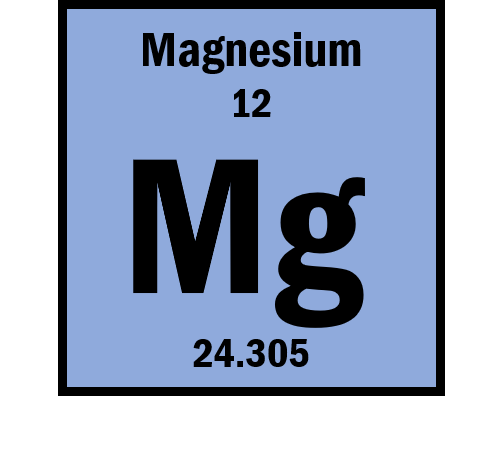
Protons=12
Neutrons=12
Electrons=12