What is an atom?
Basic building blocks of matter.
What are molecules made of?
Two or more atoms
What are elements made of?
Atoms.
Answers will vary.
Define proton.
particle inside the nucleus of an atom that has a positive charge.
What is at the center of an atom that holds the protons and neutrons?
Nucleus
Write the molecular formula for water.
H2O
True or false.
Elements are pure substances.
What does the symbol Au stand for?
Gold
Define neutron.
Neutral particle inside the nucleus of an atom.
What force holds all the parts of an atom together?
The electromagnetic force of attraction between the protons and the electrons.
Draw carbon dioxide.
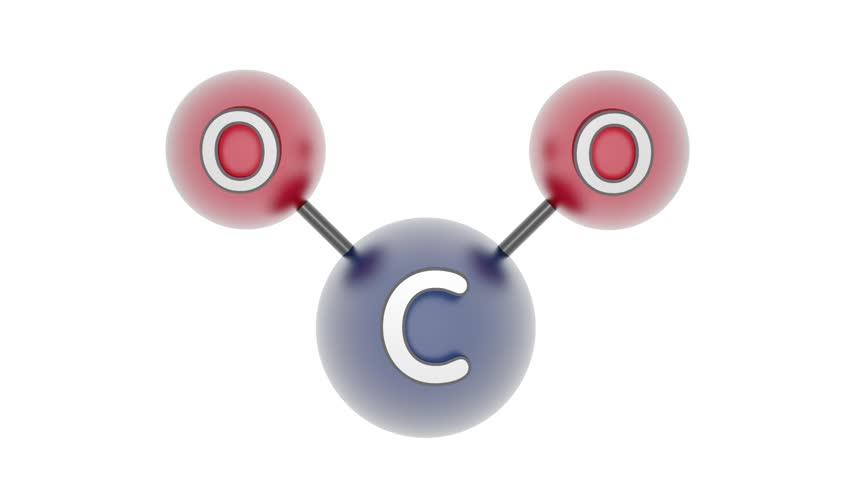
True or false.
Each element has specific number of protons in the nucleus.
True.
What tells us the number of protons and neutrons?
Atomic Mass
Define electron.
Where can you find atoms?
EVERYWHERE!
How many hydrogen molecules are in this molecule?

2
How many types of atoms are inside an element?
One.
What is the symbol for potassium?
K
Define atomic number.
Number of protons in an atom's nucleus.
Which component of an atom has negative charge?
Electron.
Name an everyday substance that is a molecule.
What element is found in every living thing?
Carbon
How many elements are on the Periodic Table?
112
Define element.
substance with atoms that are alike.