These things make up Elements.
What are Atoms?
This property of matter can be used to figure out what a foreign substance is. Jewelers are able to tell fake diamonds from real ones by doing this.
What is density?
The charge of the electons are ______
What is Negative?
The protons in Carbon atom.
What is 6?
This is how you are able to find mass.
What is a balance?
When 2 or more of the same atoms join together.
What is a Molecule?
LxWxH=
What is Volume?
The charge of Protrons are_____
What is Positive?
The Creator of the Periodic Table.
Who is Dmitri Mendeleev?
This property of a metal describes the metal's ability to shine or sparkle in light.
What is luster?
The number written above the element's sign.
What is Atomic number?
This property uses a scale.
What is weight?
_______ _______ is the number of protons found in the nucleus
What is Atomic Number?
This element has a chemical sign of Ar.
What is Argon?
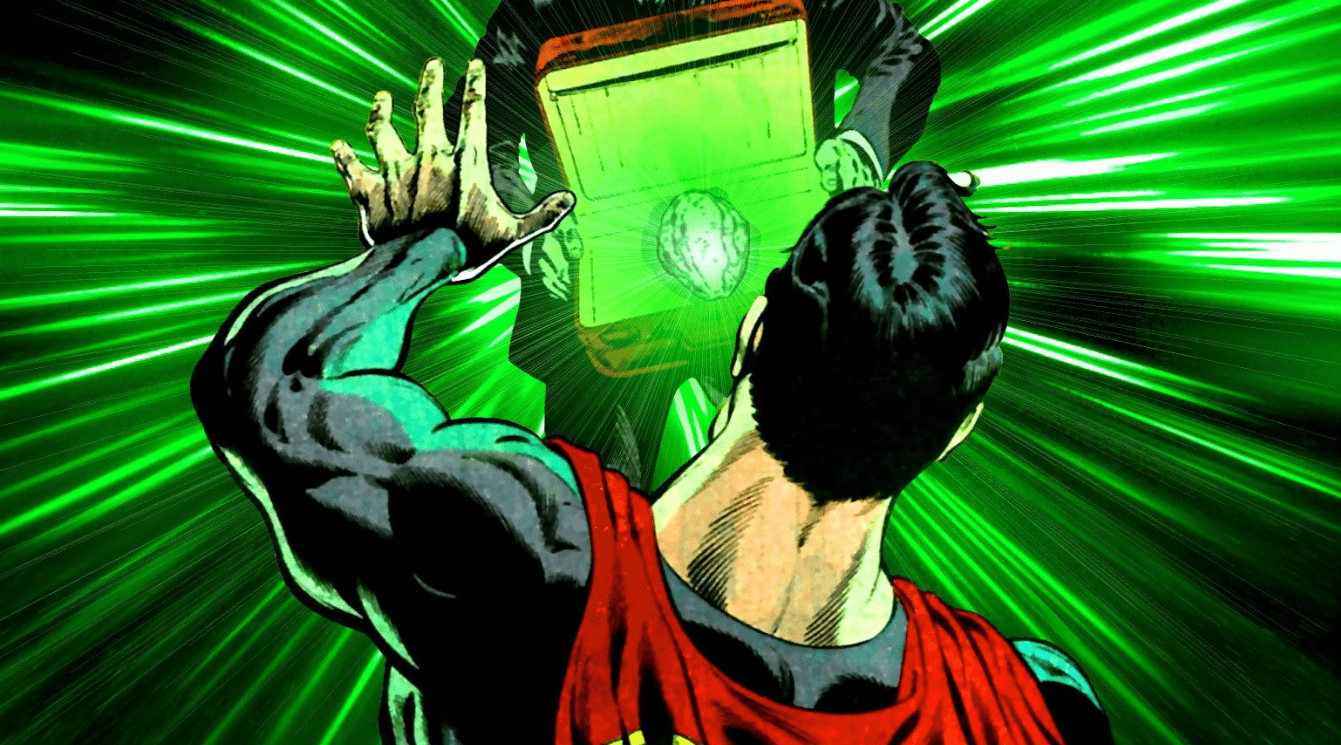
This image displays a noble gas, but also Superman's weakness.
What is Krypton?
A substance made up of only one kind of atom.
What is an Element?

This property of matter will never change. For example the __________ of an object remains the same on Earth, Jupiter and the Moon.
What is mass?
____and_____ are found in the nucleus
What is Protons and Neutrons?
The majority of the elements in the periodic table cab be classified as
What are metals?
C6H12O6 or a common house hold item.
What is glucose or sugar?
This is the joining of two different types of atoms.
What is a compound?
This property describes whether or not an object can float.
What is buoyancy?
These make up atoms.
What are Subatomic Particles?
The atomic theory states that matter is made up of
What are Atoms?
This chemical symbol is Fe or you may know its name from this famous hero. 
What is Iron?