The charge that protons have.
What is positive?
They are atoms of the same element with the same number of protons, but different number of neutrons.
What are isotopes?
These are the three (3) phases of matter.
What are solid, liquid, and gas?
These are properties of matter that can be observed without changing the chemical composition.
What are physical properties?
What are protons?
The nucleus is composed of these two subatomic particles.
What are protons and neutrons?
This is the number of neutrons that C-13 has.
What is seven (7)?
This phase of matter can spread out indefinitely when not contained, but can fit any shape when contained.
What is gas?
These properties of matter are not dependent on the amount of matter.
What are intensive properties?
This is the Bohr model for this element.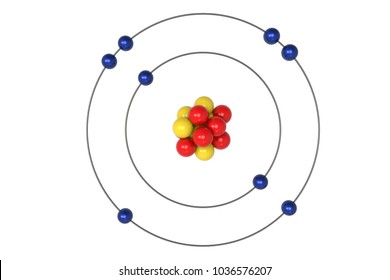
What is Oxygen (O)?
This is the term referring to the number of protons an element has.
What is atomic number?
When calculating average atomic mass, you have to multiply each atomic mass by this value.
What is abundance?
Mixtures can be classified in these two broad categories.
What are heterogeneous and homogeneous?
Volume and mass are examples of these kinds of properties of matter.
What are extensive properties?
The name of the electrons that participate in the formation of chemical bonds.
What are valence electrons?
This is the formula for calculating the number of neutrons an element has.
What is atomic mass minus the number of protons?
This is the time it takes for matter to break down by 50%?
What is half life?
This separation method separates solids from liquids or gas.
What is filtration?
Combustion and flammability are examples of these kinds of properties of matter.
What are chemical properties?
This is the number of valence electrons for Sodium (Na).
What is one (1)?
This is the number of neutrons for Potassium (K).
What is twenty (20)?
This is the process atoms go through to get back to homeostasis when unstable.
What is radiation?
This separation method separates liquids by utilizing boiling point and condensation.
What is distillation?
The rusting of a lock is an example of this chemical property.
What is oxidation?
This is the Lewis Symbol for Magnesium (Mg).
What is Mg:?