The positively charged particle
A proton
A _____ is a very small particle that is the basic unit of matter.
Atom
What is the chemical formula of water?
H2O
When looking at the Periodic Table how can you tell the difference between the Atomic Number and the Atomic Mass?
1) The Atomic Number is usually on top and the Atomic Mass is usually on the bottom
 What is the Atomic Number of Silicon?
What is the Atomic Number of Silicon?
14
The number of protons in an atom of an element is its Atomic ______
atomic number
This particle moves around the outside of nucleus
electrons
Is this molecule a compound?
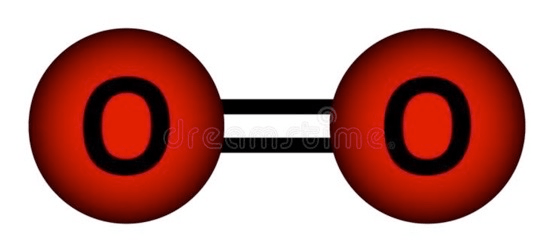
No
 How many electrons does a Beryllium atom have?
How many electrons does a Beryllium atom have?
4 electrons
What is the number of electrons in Silicon?

14
What is located in the nucleus of an atom?
protons + neutrons
Neutron have _______ charge
zero / neutral
Is Cl2 a molecule?
YES
What is the name of this specific molecule?
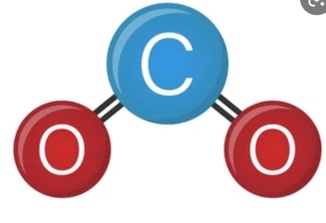
Carbon Dioxide
What is the Symbol of Silicon?

Si
How many electrons can fit in the second shell of an atom?
8
These are things that are made of two or more atoms
molecules
Which bond gives away it's electrons to create a bond?
Ionic bond
A ________ is a group of chemically bonded atoms. They can be of the same element or different elements.
What is the number of neutrons in Zinc?
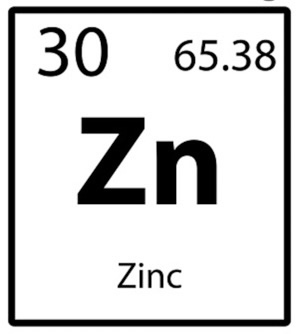
35 or 35.38
What two particles determine the overall charge of an atom?
protons and electrons
The most dense structure within an atom is called the _______ and it is made up of ______ and ______.
nucleus - neutrons and protons
When electrons are shared as atoms bond, a _________ bond is formed.
Covalent
What chart lists every known atom/element and what is the first element listed?
The Periodic Table; Hydrogen
What is the Atomic Number, Atomic Mass and Number of Protons, and Electrons in Cobalt?

Atomic Number: 27
Atomic Mass:58.933 or 59
# of protons: 27
# of electrons: 27