The smallest unit of any chemical element
What is the atom?
The table that organizes all known elements.
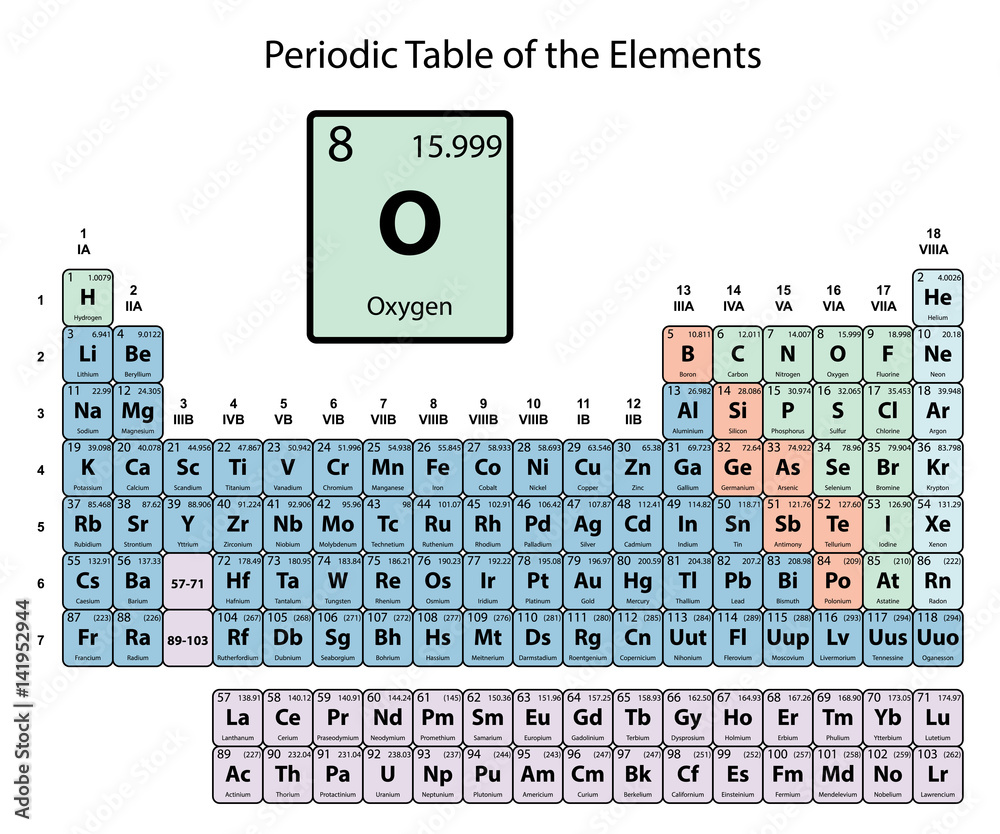
What is the periodic table.
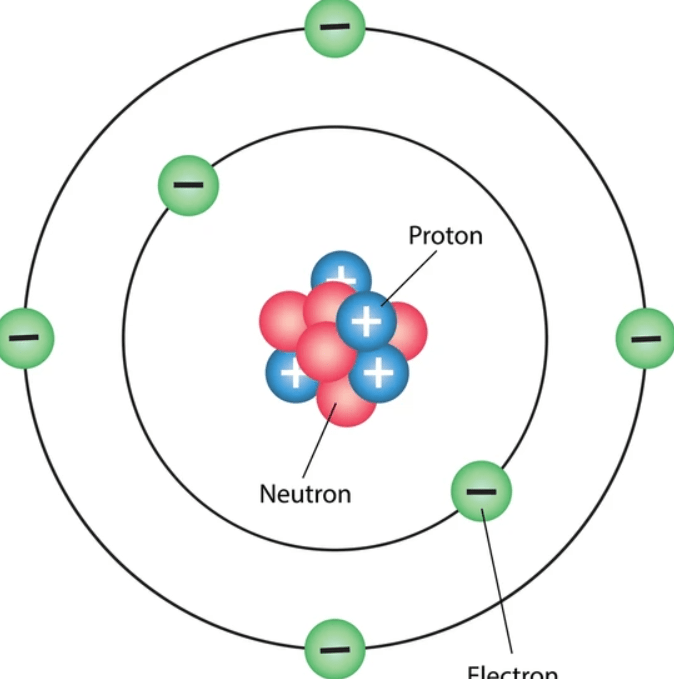
The smallest subunit of an atom - has an electric charge
What is an electron?
The following element's atomic number.

What is 8?
The element that has 6 protons, 6 neutrons, and 6 electrons.
What is Carbon?
Group of elements that are poor conductors, lack luster, and are typically brittle solids or gasses at room temperature.
What are non-metals?
The property of some non-metals tat describes its fragile composition at room temperature and likeliness to fall apart.
What is brittle?
One of the two larger subunits of an atom. It has a positive charge.
What is a proton?
The following element's atomic symbol.
What is "N"?
The property of metals that allow them to transfer electricity easily.
What is conductivity?
The group of elements that show traits of both metals and non-metals.
What are metaloids?
The number that designates the how many protons are found in an element. Also identifies the element itself.
What is the atomic number?
One of the two larger subunits of an atom. It has a neutral charge.
What is a neutron?
The following element's atomic mass.
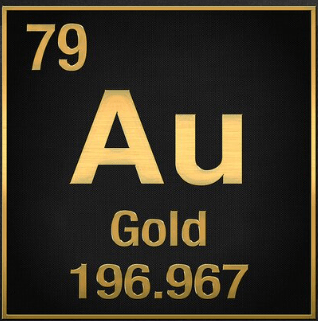
What is 196.967?
If I were to add more of this atomic subunit, the atom would gain mass but not change in charge.
What is a neutron?
Trait describing a metal's ability to be hammered or pressed permanently out of shape without breaking or cracking.
What is malleable?
Indicates weather an atom is positive or negative.
What is the charge?
The electrons found in the outermost shell of an atom.
What are valence electrons?
The following element's name?

What is Cobalt?
The group of elements found in the rightmost column of the period table. Non-reactive.
What are the nobel gasses?
Distinguished by its atomic number, this is a pure substance made of only one type of atom. It cannot be broken down into simpler substances by chemical means.
What is an element?
Trait describing a metal's ability to be drawn out into a thin wire.
What is ductile?
The average mass of an atom after the mass of neutrons, protons, and electrons are added up.
What is the atomic mass?
The following element's average number of neutrons.

What is ~30?
(55.8 - 26 = 29.8, then round that to 30)
Calculated by subtracting the atomic number from the atomic mass.
What is the average number of neutrons?