What are the three parts of an atom?
What are protons, neutrons, and electrons
What are the three main parts of the periodic table?
Metals, non-metals, and metalloids
What does the period number equal in an atom?
the energy levels
How many valence electrons does this atom have?
1
What about an atom determines if it is reactive or stable?
The number of valence electrons
The charges of protons are _______ and neutrons are ___________
positive, neutral
Which of the three major parts of the periodic table conducts electricity and is malleable.
Metals
What does the group number tell you about an atom?
The number of valence electrons
What atom is this?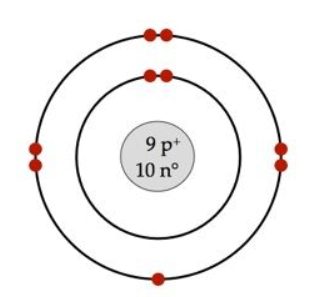
Fluorine
Double Jeopardy!!
Is this a metal, non-metal, or metalloid?
What is the most stable group in the periodic table?
Group 18, the noble gases
The nucleus contains which particles?
Protons and neutrons
What part of the periodic table would an element be that is not malleable but can conduct electricity?
Metalloids
Which list of elements have similar properties?
List 1: Boron, Helium, Galium
List 2: Oxygen, Sulfur, Selenium
List 3: Lithium, Beryllium, Aluminum
List 2 because they are in the same group
What group is this atom in?
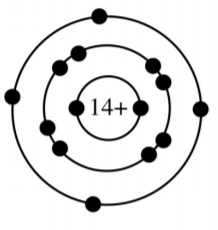
Group 14
Is this atom reactive or stable?
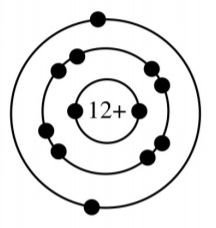
Reactive
What part of the atom has the most volume or takes up the most space?
The electron cloud
These elements are not ductile and are dull
Non-metals
Members of the same group are also called families because
They react in a similar way, have the same number of valence electrons
What are the period and group numbers for this atom?
Group 1 Period 4
Is this atom reactive or stable?
Stable
Which of these has a positive charge?
Electrons
Protons
Neutrons
Nucleus
Electron Cloud
Protons and Nucleus
What is the property that allows an element to be wound into a wire without breaking?
Malleable, ductile
Which group is the most reactive:
Group 1
Group 7
Group 8
Group 1
How many energy levels and valence electrons does this atom have?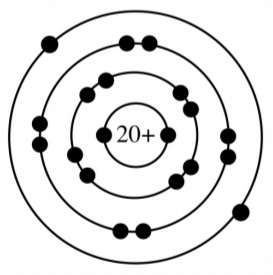
3 energy levels, 2 valence electrons
Double Jeopardy!!!
What is this atom and what is the only thing that identifies an atom?
Which group is more reactive?
Group 3
Group 15
Group 17
Group 17
Double Jeopardy!!!
What is the most reactive group in the periodic table?