the 3 subatomic particles
proton, neutron, and electron
How many valence electrons do group 2 elements have?
2
These are vertical columns on a periodic table
groups
How many protons are in the following element?

36
What is the difference between a Period and a Group on the Periodic Table
Groups are the vertical columns where elements share the same number of valence electrons and similar chemical properties, while Periods are the horizontal rows where elements have the same number of Energy Levels
the location of protons and neutrons
the nucleus
This number tells you the number of protons
the atomic number
These are horizontal rows on a periodic table
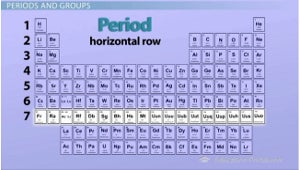
periods

How many Protons does this element have?
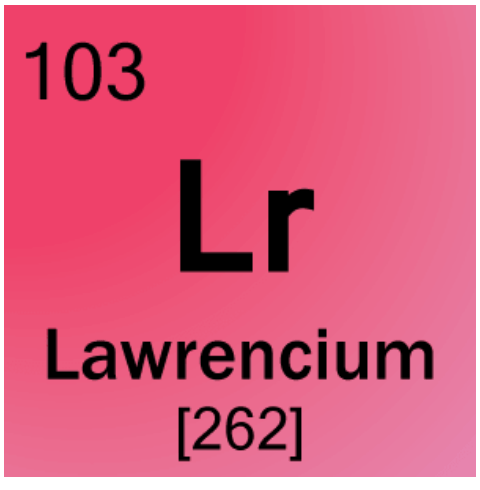
103 Protons
Which particle has the lightest mass
electrons
The atomic number is equal to these positive particles
protons
An element is in period 3, group 18 and in the noble gas family. Would it be classified as a metal, nonmetal or metalloid?
nonmetal
The atomic number tells us how many __________ an element has in its nucleus.
protons
Which element is in period 2 and is an alkali metal
Lithium (Li)
The electrons outside an atom's nucleus have what kind of charge?
Negative Charge
An element is in period 3, family 14. How many energy levels would the Bohr model of this element have?
3
![]()
Which makes up majority of the periodic table?
Metals, Non-Metals or Metalloids
Metals
How many neutrons does the following element have?

16
How many electrons in the outer shell of Chlorine and which Period is it found in?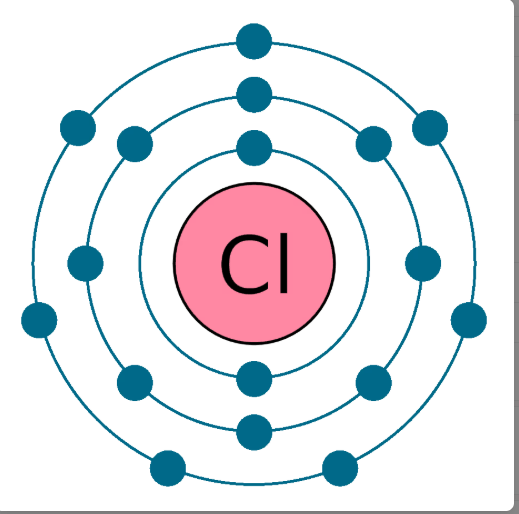
7 Electrons and Period 3
How many total Atoms are there?

17 Atoms
the outermost electrons
valence electrons
This is the number of neutrons in the element Cl with the Atomic Number 17 and the Atomic Mass of 35.453 u(chlorine).
18 Neutrons
An atom of an element with atomic number 48 and atomic mass of 112 contains
48 Electrons, 48 Protons and 64 nuetrons
How many Atoms are in both formulas:
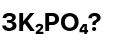
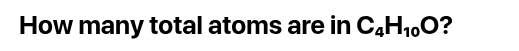
21 Atoms
15 Atoms
Silico is shiny, yet, brittle. It allows some electricity to pass through it, but not too much, which means it is a semiconductor. Is silicon a metal, nonmetal or metalloid?
metalloid