What is the element with the atomic number of 30?
Zinc
The number of electrons is the same as the number of:
Protons
Neutron is _______ charged
Uncharged/ neutral
What is the name of this element
Potassium
What does a nucleus contain?
Protons and neutrons
 How many atoms of carbon are in this molecule?
How many atoms of carbon are in this molecule?
1
How many protons and electrons does this element have? Atomic number: 4
4 protons, 4 electrons
The positively charged particle
Proton
electrons are _____ charged
Negatively
What does potassium represent?
Name of the Element
True or False? The material that gains electrons becomes negatively charged.
True
How much helium does this molecule have?
How many neutrons does an element with an atomic number of 21?
About 24 (23.956)
The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the _____
Atom
Electrons transferring from one place to another creates _____ electricity
Static

What does 19 represent?
The Number of Protons
 Are electrons inside or outside the nucleus?
Are electrons inside or outside the nucleus?
outside
How much oxygen is in this molecule?
1
What is the atomic mass of a molecule with the atomic number of 85?
210
In an atom, which part has the least mass?
Electron
The sum of protons and neutrons in the nucleus is the
Atomic mass

What does 39.098 represent?
The Atomic Mass
What part of a molecule can move around from one atom to another?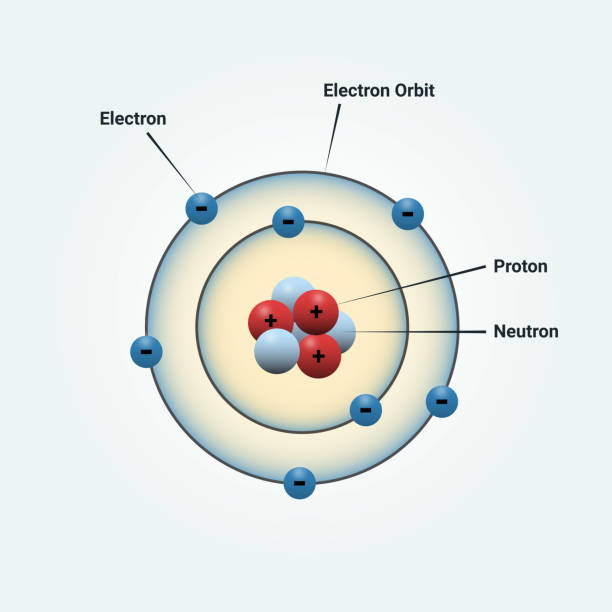
Electrons
What element is this?
Hydrogen
What element has the atomic number of 105?
(trick question)
No element
Which is bigger; the proton or the electron?
Protons are _____ charged
Positively

What does K represent?
Atomic symbol
 What is a molecule and how do you recognize one?
What is a molecule and how do you recognize one?
Atoms mixed together to make a new element, a clump of atoms that are clumped together
What element is this?
Carbon