The number of protons in an atom of an element is its ___________ number.
What is atomic?
A positively charged subatomic particle.
What is a proton?
The number of protons in this element.
What is 5?
Atomic Number

7
Protons and neutrons can be found in the _____________ of an atom.
What is nucleus?
A (n) _____ is a very small particle that is the basic unit of matter.
Atom
A negatively charged subatomic particle with an amu of 1/2000.
What is an electron?
The number of neutrons in this element.

What is 6?
Electrons

2
Determines the identity of an element.
What is the number of protons or atomic number?
Two subatomic particles that make up the net charge of an atom's nucleus?
What is protons and electrons
__________ is the basic building block of all matter.
What are atoms?
The atomic weight of this element.
Atomic Mass

The atomic number of the element equals what two subatomic particle numbers?
Protons and Electrons
Electrons in an atom move throughout the electron __________ surrounding the nucleus.
What is cloud?
A subatomic particle that has no charge.
What is a neutron?
The number of electrons in this element.
What is 79?
Element
Oxygen
A fluorine atom has 9 protons, 10 neutrons, and 9 electrons. What is the atomic number?
9
True or False. The smallest sub-atomic particle is a proton.
False
The whole number that is in the upper portion of each element on the periodic table, it is also the number of protons that element has.
What is the atomic number?
The number of neutrons in this element.
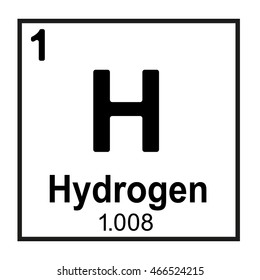
What is zero?
Atomic Mass

23
Potassium has 19 protons and an atomic mass of 39. How many neutrons does potassium have?
20
Draw the atomic structure of Carbon. Be sure to label protons, neutrons, electrons, and nucleus.
There should be 6 protons, 6 neutrons inside the nucleus along with two orbitals. The first orbital will have 2 electrons and the second orbital will have 4.
