in an atom, the number of protons equals the number of :____
electrons
A subatomic particle that has a positive charge and that is found in the nucleus of an atom
proton
which particle of an atom has a negative charge ?
electrons
True or False: nonmetals can be found on the right side of the periodic table.
(if false name correct answer)
True
compare the properties of metals and non-metals:____
(list at least 2)
NON METALS: dull, gases at room temperature, solid non metals are usually dull and brittle
METALS: solid at room temperature, shiny, malleable, good conductors of electricity
The rows in the Periodic Table are called this
Periods
How many of each atom are there in the chemical formula: H2O
Two Hydrogen and 1 Oxygen
a chemical bond formed when two atoms share electrons is called:____
covalent bond
A subatomic particle that has no charge and that is found in the nucleus of an atom
Neutron
which information in the periodic table indicates the number of protons in an atom ?
atomic number
True or False: the horizontal row in the periodic table are known as groups.
(if false name correct answer)
False, periods
how do valence electrons relate to the chemical reactions of an element?
chemical reactions occur whenever valence electrons are shared or transferred between atoms. show how strong it would be.
The columns in the Periodic Table are called this.
Groups/Families
How many of each atom in the chemical formula
6NaF
6 Sodium and 6 Fluorine
when an atom loses an electron, it becomes a:____
positive ion
The number that identifies an atom of an element
Atomic Number
Number of protons and neutrons
Atomic Mass
(if false name correct answer)
False, atomic number
compare a covalent and an ionic bond:_____
(at least two)
COVALENT BOND: two atoms share valence electrons. neither atom loses electrons or takes electrons from the other.
IONIC BOND: one or more electrons are transferred from one atom to another. atoms that lose electrons become positively charged ions, atoms that gain are negatively charged.
The left side of the periodic table is filled with this type of element
Metals
How many of each element in Mg(NO3)2
1 Magnesium, 2 Nitrogen, 6 Oxygen
electrons involved in bonding between atoms are:_____
valance electrons
What is label C pointing at and who discovered it
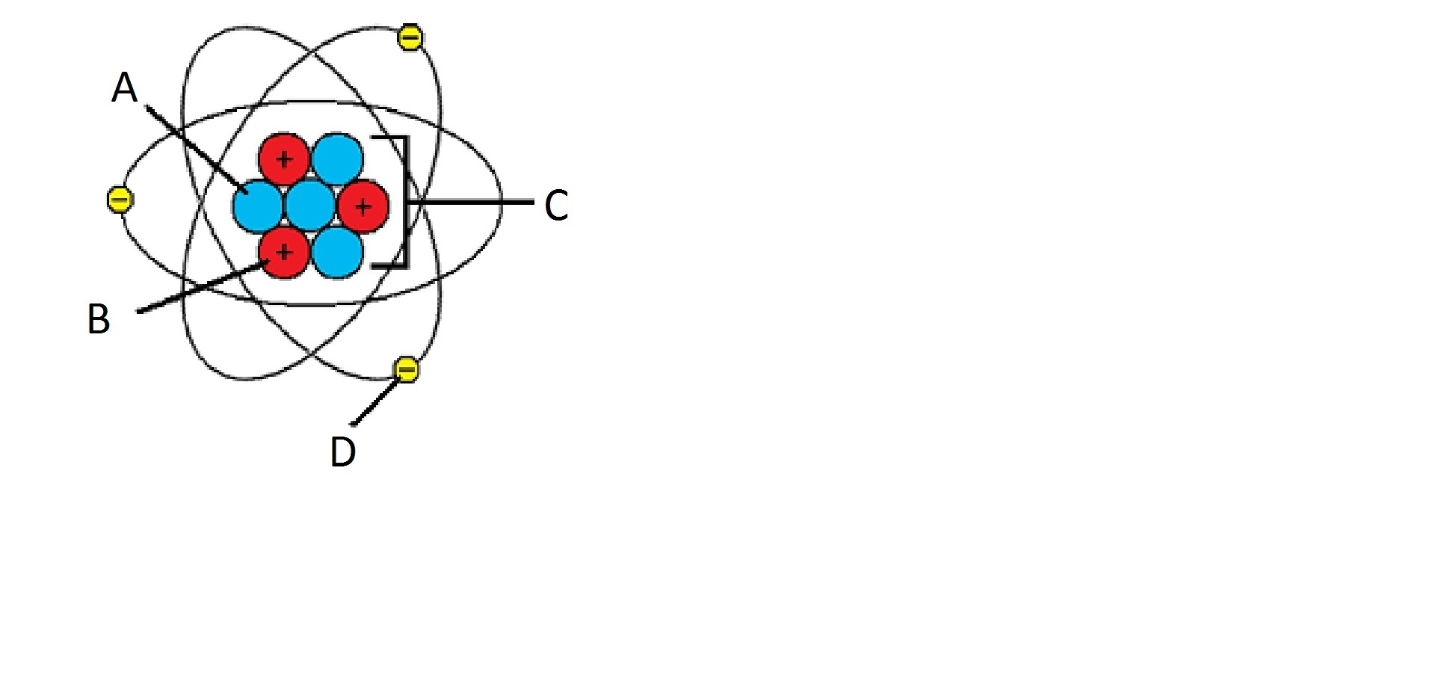
The nucleus, Rutherford
how many sodiums are in this formula?
4Na2S
8
True or False: atoms with the same number of protons and different numbers of neutrons are called isotopes
(if false name correct answer)
True
the mass of an atom may be determined by adding the masses of the protons and neutrons in the nucleus. why is it unnecessary to include the electrons when determining the mass of an atom ?
the mass of an electron is much lower than the mass of a proton or a neutron. when adding the masses of the particles together, the mass of electrons will not add significant amount to the total mass of an atom.
Boron, Silicon and Polonium are all examples of this type of material and have these properties.
Metalloids. Can act as a metal and a non metal at the same time.
How many of each element in
7Al2(SO4)3
14 Aluminum, 21 Sulphur, 84 Oxygen
measurements of half life make radioactive isotopes useful for:______
determining the aging of rocks and fossils
The amount of valence electrons and energy levels in Silicon
4 Valence electrons, 3 electron shells
true or false: transition metals contain the most elements on the periodic table?
(if false name correct answer)
false, metals
True or False: when electrons are transferred between two atoms, a covalent bond is formed
(if false name correct answer)
False, shared
in an electron dot diagram of aluminum (Al), how many dots should be drawn around the elements symbol ?
3 valence electrons, elements in group 13 have 3 valence electrons.
In the periodic table, the most reactive metals are found:_______
name one metal:_____
group 1, the first column on the left
How much sodium and boron is in the formula?
2NaMg3Al6(BO3)3Si6O18(OH)4
2 Sodium, 6 Boron