This is a small particle that makes up all elements that exist on the earth
What is an atom
This is the sum of the an atom's protons and neutrons
What is MASS NUMBER
All elements in the same group of the periodic table have this in common _________ and all elements in the same period have this in common __________.
Number of valence electrons,
Number of electron shells
This is the number of protons in the nucleus of each atom of an element
What is ATOMIC NUMBER?
These are the vertical columns on the Periodic Table
What are Groups?
What happens to the atomic radius of elements as a group is descended?
What is INCREASE
 A nuclear notation is presented above. What is the significance of the numbers and letter presented?
A nuclear notation is presented above. What is the significance of the numbers and letter presented?
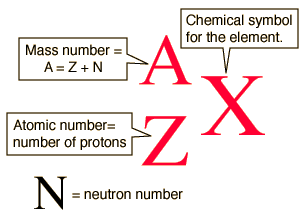
N represents the chemical symbol of nitrogen
14 is the mass number that totals the protons and neutrons
7 is the atomic number showing the number of protons
The horizontal rows on the Periodic table are called ______________.
What are PERIODS?
What is INCREASE?
How many neutrons are present in the hydrogen atom below?
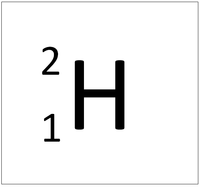
What is ONE NEUTRON?
State the family name given to the following groups on the periodic Table.
Group 1
Grroup 2
Group 7
Group 0
Group 1 - alkali metals
Group 2 - alkaline earth metals
Group 7 - halogens
Group 0 - noble gases
Which group 7 element is most reactive?
Fluorine
Bromine
Chlorine
Iodine
Fluorine
A charged atom is presented below.

State the name of this type of charged particles and describe how the beryllium formed this charged particle
What is a CATION?
Beryllium forms a cation by losing 2 electrons from its valence shell. 
An element has 16 electrons in total, state its electronic configuration and identify the group and period it belongs to on the periodic table.
Electronic Configuration: _____________?
Group #: ______________?
Period #: ______________?
Electronic Configuration: 2,8,6
Group #: Group 6
Period #: Period 3
TRUE OR FALSE
Group 2 elements react with dilute hydrochloric acid to produce metal chlorides and hydrogen gas.
What is TRUE?