Charges of each subatomic particle.
Protons: Positive, Neutrons: Neutral, Electrons: Negative
Isotopes are atoms of the same element that have different numbers of these.
What is neutrons?
True or False. There are 18 Groups and 7 Periods on the Periodic Table.
What is True?
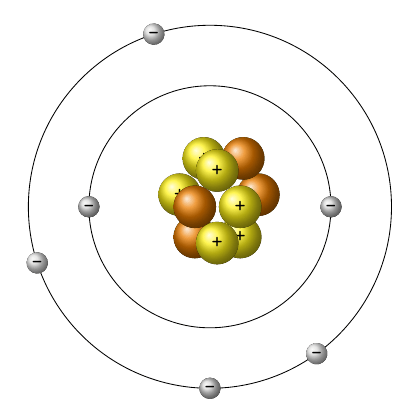
Label each subatomic particle in the Bohr Model: orange, yellow, silver.
What is...
Yellow: protons; Orange: neutrons; Silver: electrons
The trend of electronegativity as you go across a period versus down a group
What is increasing versus decreasing?
Information that the atomic number can tell you.
What is number of protons, number of electrons (if it's neutral), and identity of the element?
Definition of an ion and its two names with the charges
What is a charged particle that either loses or gains electrons, cation (+), anion (-)
This noble gas has only two valence electrons, instead of the usual eight.
What is Helium (He)?
The dots on a Lewis dot structure are what aspect of the element?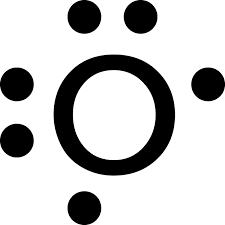
What is valence electrons?
True or False. Strontium has a smaller atomic radius then Gallium.
What is False?
The mass number of an atom is calculated using these two subatomic particles.
What are protons and neutrons?
True or False. Anions are typically metals and cations are typically nonmetals.
What is false?
Elements in the same group have the same number of these.
What are valence electrons?
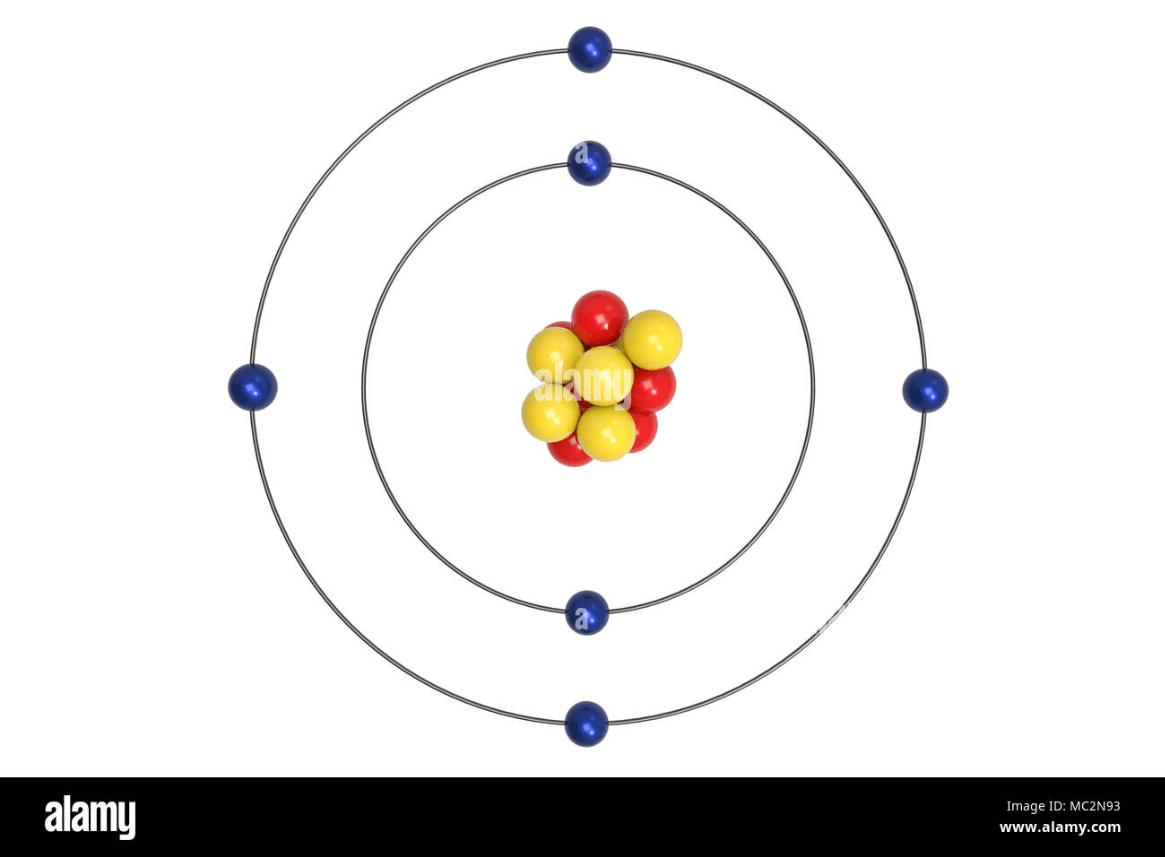
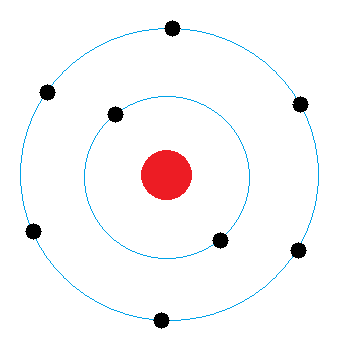
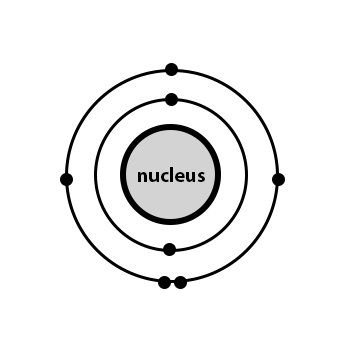
Determine Oxygen.
What is the middle diagram?
Between oxygen and tin, this element has the higher electronegativity.

Determine the element and the number of each subatomic particle.
What is...
Carbon, 6 protons, 6 neutrons, 6 electrons
The charge of Germanium and whether it will gain or lose electrons.
What is...
+4, lose electrons
This rule states that atoms tend to gain, lose, or share electrons to have a full outer shell.
What is the Octet rule?
What Lewis Dot structures match Sulfur.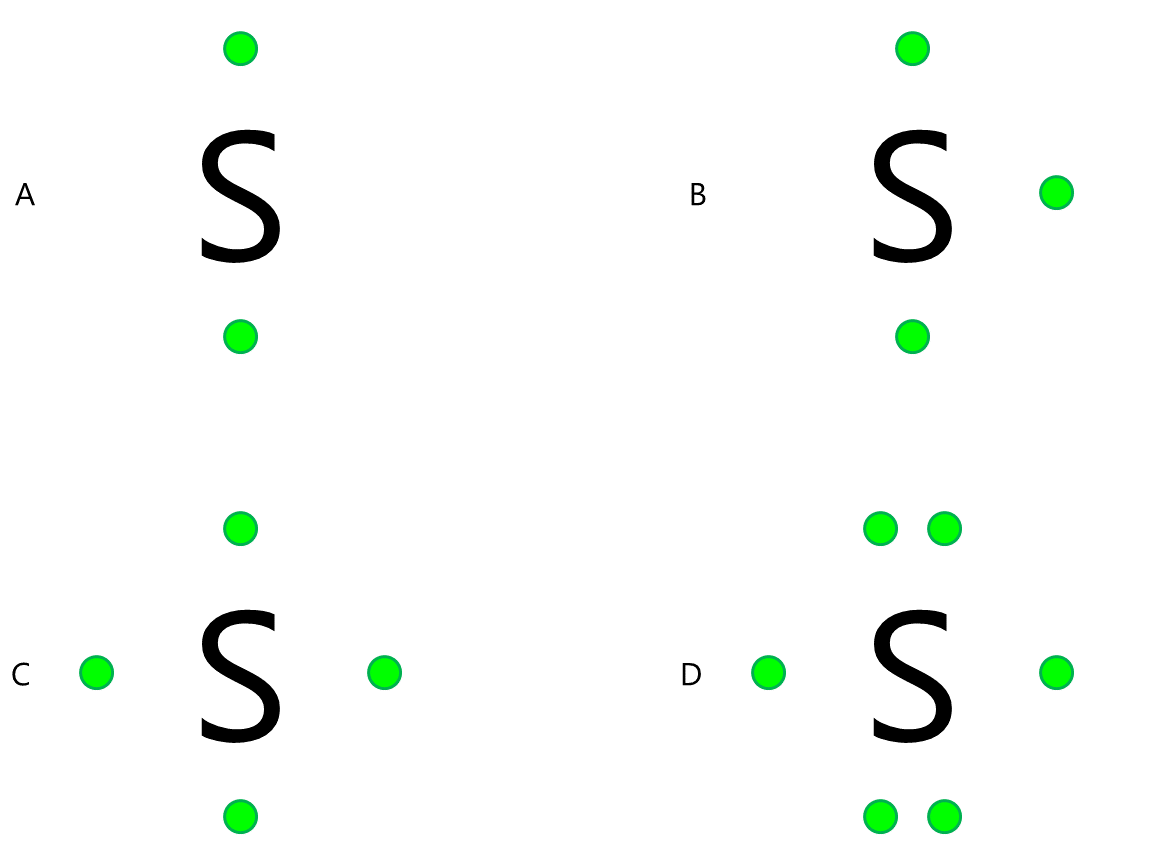
What is D?
Order the following elements from smallest atomic radius to largest atomic radius:
Oxygen (O)
Francium (Fr)
Silicon (Si)
What are O, Si, Fr?
The periodic table is arranged in order of increasing this atomic property.
What is atomic number?
The isotope carbon-14 has this number of neutrons, protons, and electrons
This element is in Group 15, Period 3. What is the element and how many valence electrons does it have?
Phosphorus; 5
Draw the Lewis Dot Structure for Silicon.
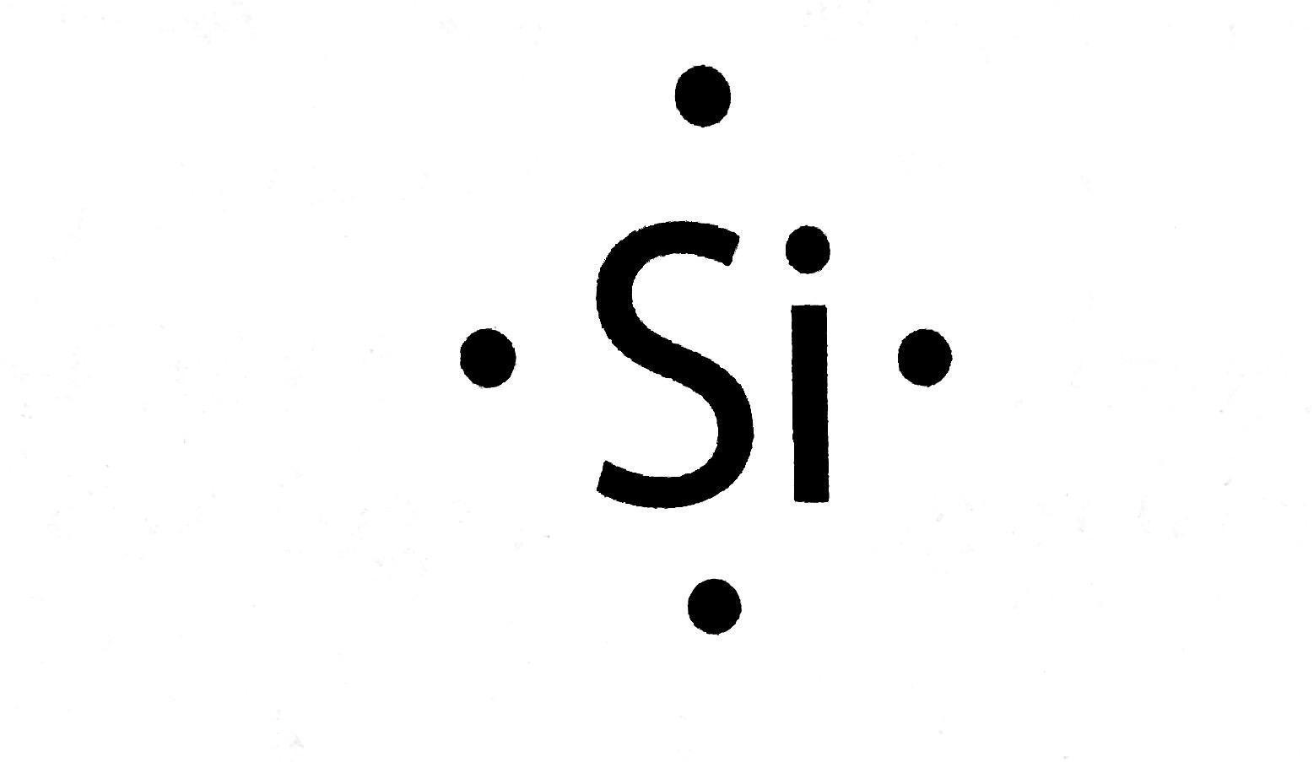
Order the following elements from smallest to greatest ionization energy:
Magnesium (Mg)
Oxygen (O)
Sodium (Na)
Chlorine (Cl)
What is Na, Mg, O, Cl?