the center of the atom is called the _______________
nucleus
The Periodic Table of Elements
What does the atomic number tell you about an atom?
the number of protons
What does the atomic mass of an element tell you?
the total number of protons and neutrons in the atom
I have 6 protons, 6 electrons, and 6 neutrons. What atom am I? I'm found in all living things
Carbon
what two subatomic particles are located in the nucleus?
protons and neutrons
How are the elements on the Periodic Table arranged?
The are arranged based on their properties and characteristics - they are in rows based on their # of protons and the columns represent their electrons and reactivity
How do I calculate the number of neutrons in an atom?
Two strategies can be used
1. protons + neutrons = atomic mass
2. atomic mass - atomic number = neutrons
Why are most of the atomic numbers on the Periodic Table listed as decimals and not whole numbers?

Most atoms can found with different numbers of neutrons in their nucleus - (ex. Some Lithium atoms have 3 neutrons and some have 4 neutrons. This is why the atomic mass is 6.94)
I have 17 protons and 18 neutrons - I am often used as a disinfectant in water. I appear as a yellow gas at room temperature. What element am I?
Chlorine
More neutrons are needed to help keep the atom stable (the larger the atom, the harder it is to keep stable)
Identify the charge of the each of the items below
-protons
-electrons
-neutrons
protons = positive
electrons = negative
neutrons = no charge
A copper atom has 29 protons an an atomic mass of 64. How many neutrons does it have
35 neutrons
A Zirconium atom has 40 protons an an atomic mass of 91. How many neutrons does it have
51 neutrons
I have 80 protons and 121 neutrons - I am used in electrical and electronic equipment, such as fluorescent lamps and switches, and in scientific and medical instruments like thermometers and barometers.
I am the only metal that is a liquid at room temperature. What element am I?
Mercury
For an atom to be considered balanced (no charge), what does it need?
It needs the same number of protons and electrons (this is because the positive and negative charges each other out)
Label the atom in the image below
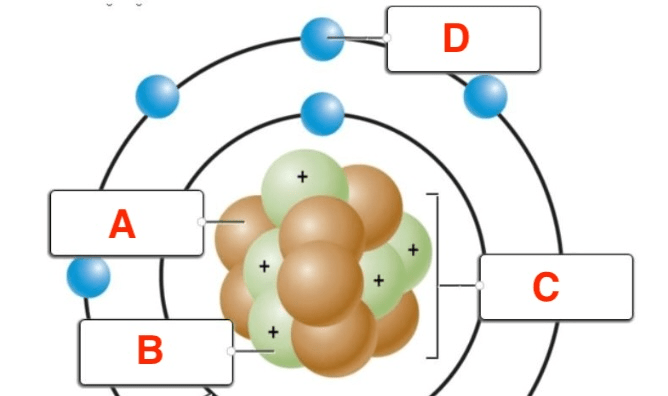
A - Neutrons
B - Protons
C - Nucleus
D - Electrons
How many protons, neutrons, and electrons does this Chlorine atom have?

17 protons
17 electrons
18 electrons
Calculate the protons, neutrons, and electrons for this Krypton atom

36 protons
36 electrons
48 neutrons
I have 53 protons and 74 neutrons - I am a solid at room temperature. I am used in many health and medical remedies. What element am I?
Iodine
List three specific facts about electrons
1. they orbit the nucleus in shells
2. they have very little mass
3. they move at the speed of light
4. they are negatively charged
What is the difference between an element an a molecule?
an element is substance made of all of the same type of atoms; a molecule is when different types of atoms bond together to create a new substance
Calculate protons, neutrons, and electrons for this Nickel atom
![]()
28 protons
28 electrons
31 neutrons
How many protons, electrons, and neutrons does this Gold atom have?
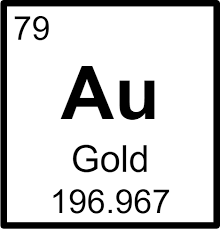
79 protons
79 electrons
118 neutrons
I have 92 protons and 146 neutrons. I am a radioactive metal found in rocks, water, and soil. I am primarily used in nuclear reactors for electricity generation and in military applications like nuclear weapons and armor-piercing bullets. What element am I?
Uranium