_______is the name of the central part of the atom
nucleus
How many total Carbon atoms are in this molecule?
5C5H10
25
Answer: (5x5)= 25
What is the mass of an atom with 4 protons, 5 neutrons, and 4 electrons?
9
Because you don't count electrons, they are too small to really affect the mass
Atom or Molecule?
Double point option: identify whether it is an atom or molecule AND what it's name is. If you get both right, you can double your points
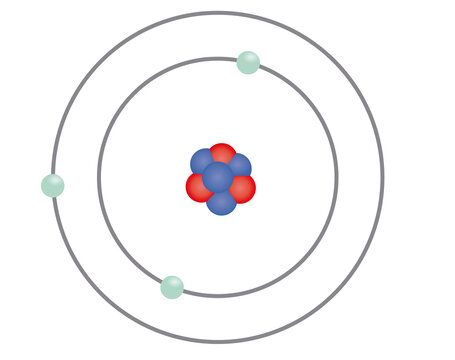
Atom
Double point answer: Lithium
If you get both right, you can double your points
This is the smallest unit of matter
atom
_____ the part of the atom is located in the nucleus and has a positive charge
proton
How many total Hydrogen atoms are in this molecule?
3LiC5H3N2O4
9
Answer: 3x3=9
This is the name of the atom with 8 electrons?
Oxygen
Atom or Molecule?
Double point option: identify whether it is an atom or molecule AND what it's name is. If you get both right, you can double your points

molecule
double point answer: Methane
If you get both right, you can double your points
Which of the following shows the atoms present in a molecule?
a. Atomic mass
b. chemical formula
c. Molecule name
d. All of the above
b. chemical formula
______ part of the atom that is outside of the nucleus. This particle has a negative charge
electron
How many TOTAL atoms are in this molecule?
C6H6MgO7
20
Answer: 6+6+1+7=20
This atom has 17 protons, 17 neutrons, and 17 electrons.
Chlorine
True or False: the majority of the periodic table is made up of gases
False
Answer: it's actually made up of mainly metals
This is a singular building block of matter
element or atom
____ part of the atom that contributes to the mass of the atom and has no charge
neutron
How many TOTAL atoms are in this molecule?
4KMnO4
24
Answer: (4x1)+(4x1)+(4x4)= 24
An atom has a mass of 55.85 and an atomic number of 26. How many protons does this atom have?
26

atoms of the same element that have different numbers of neutrons are called______
isotopes
2 or more of the same atoms combine to form ____
molecules
_______ ________ or ________ is the area surrounding the nucleus where the electrons are found
electron cloud or orbitals
How many TOTAL atoms are in this molecule?
6Na2B4O
42
Answer: (6x2)+(6x4)+(6x1)= 42
This atom has a mass of 65.4 and an atomic number of 30. How many neutrons does this atom have?
35.4 or 35
When _____ form, the atoms lose their individual properties and take on new properties.
molecules
2 or more different atoms combined to form a ______ _______
compound molecule