How many atoms are in this formula?
H2O
Three atoms:
Hydrogen = 2
Oxygen = 1
What is on the periodic table?
Elements
Which is not a molecule? C2, He, NH3
He = Helium
Which is not a compound? CO, H2, NH3, CH4
H2
What are pure substances that are not able to be broken down chemically called?
How many atoms are in this formula?
Na2
2 atoms
Na = 2
C, H, N, and O stand for what elements?
Carbon, Hydrogen, Nitrogen, and Oxygen
How many molecules are in this formula?
C4H10 + O2 --> CO2 + H20
Four molecules
How many compounds are in this formula?
CO2 + H2O -> C6H12O6 + O2
Three compounds
CO2, H2O, C6H12O6
O2 is a molecule
What are the basic building blocks of matter called?
Atoms
How many atoms are in this formula: C4H10
14 atoms
How many elements are in this formula?
Ca3(PO4)2
3
How many molecules are in this formula?
Al + H2SO4 --> Al2 + H2
Three molecules
Is this a molecule or compound?
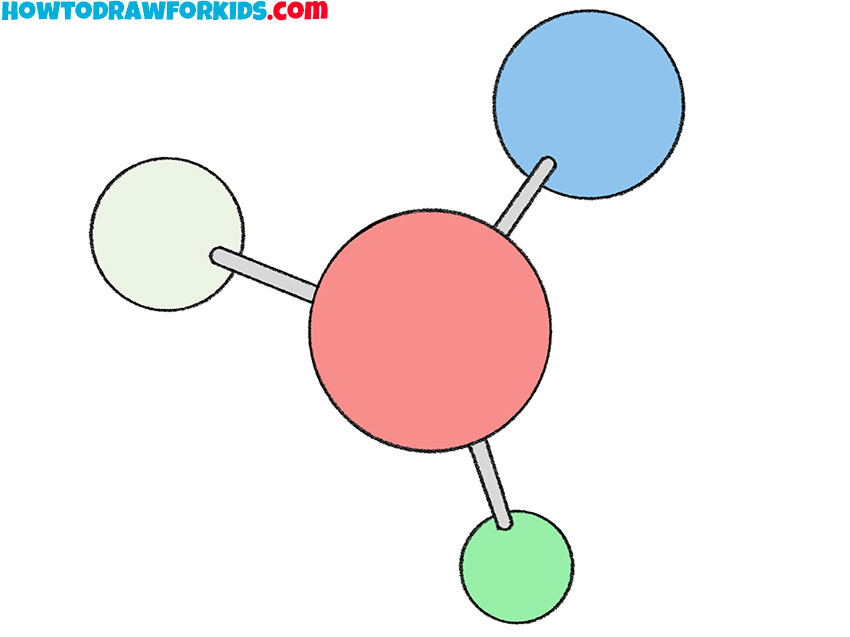
Compound
Give me an Example of a Molecule.
O2-Oxygen (we breath)
O3-Ozone
H2O-Water
H2SO4-Sulfuric Acid
CO2-Carbon Dioxide
How many atoms are in this formula: H2SO4
7 atoms.
Hydrogen = 2
Sulfur = 1
Oxygen = 4
How many elements are in this formula: C7H5(NO2)3
4
How many molecules are in this chemical?
3Cl2
3 molecules
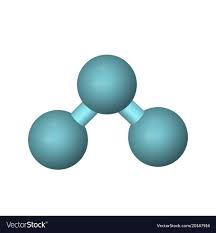 Is this a compound?
Is this a compound?
No, because all atoms are the same.
Two or more atoms of different elements that are chemically combined is called a
Compound
How many atoms are in this formula: 2(CaH2PO4)
16 atoms
Ca=2, H=4, P=2, O=8
How many atoms and how many elements are in this formula?
HgBr2
3 atoms, 2 elements.
What is the difference in a compound and a molecule?
How many compounds are in this formula?
CH4 + Cl2 --> CCl4 + HCl
Three compounds
True/False:
Compounds cannot be chemically broken down, while elements can.
False