Which subatomic particle has a positive charge and is part of the nucleus?
Proton
What is capital letter on the periodic table for each element called?
Symbol
Which is not a molecule? C2, He, NH3
He = Helium
Which is not a compound? CO, H2, NH3, CH4
H2
What are pure substances that are made of one type of atom and not able to be broken down chemically called?
Elements
Which subatomic particle orbits the nucleus and has a negative charge?
Electron
The atomic number tells us how many of what is in the nucleus of the atom?
Protons
How many molecules are in this chemical formula?
C4H10 + O2 --> CO2 + H20
Four molecules
How many compounds are in this formula?
CO2 + H2O -> C6H12O6 + O2
Three compounds
CO2, H2O, C6H12O6
O2 is a molecule
What are the tiny particles that make up all matter called?
Atoms
Which 2 subatomic particles make up the nucleus?
Protons and Neutrons
How many elements are in this formula: C7H5NO2
4
True or False: These are 4 facts about a water molecule.
1. Water molecule is H2O
2. Water molecules have 2 hydrogen atoms.
3. A water molecule has 3 atoms total.
4. The atoms of a water molecule are bonded together.
TRUE
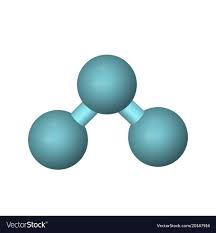 Is this a compound?
Is this a compound?
No, because all atoms are the same.
Two or more atoms of different elements that are bonded together is called a _________.
Compound
How many TOTAL atoms are in this formula: C4H10
14 atoms
How many elements are in this formula?
NaCl
2
Chemical Bonds
Is this a molecule or compound?
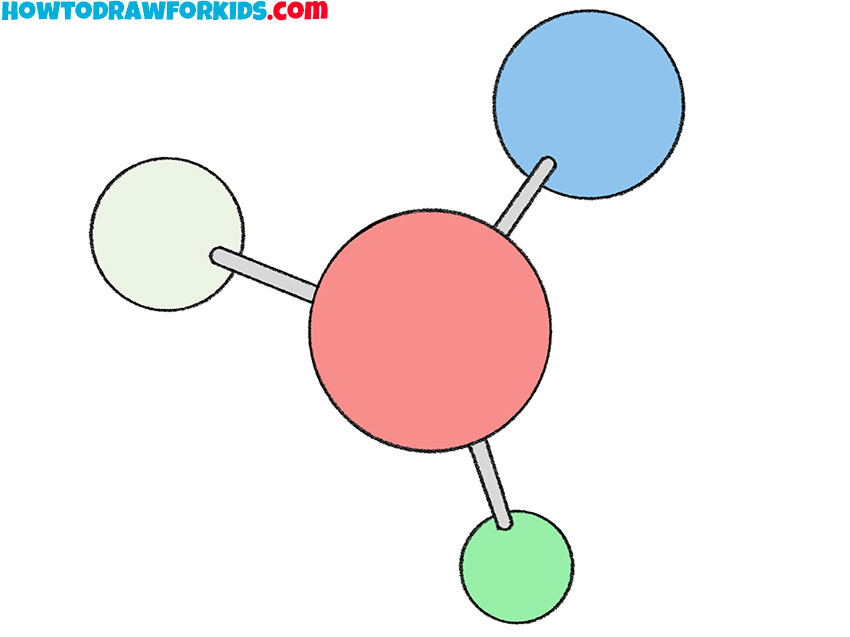
Compound
A ________ __________ is the visual representation of what is happening in a chemical reaction.
Chemical Equation
How many atoms of each element are in this formula: CaH2PO4
Ca = 1
H = 2
P = 1
O = 4
How many atoms and how many elements are in this formula?
HgBr2
3 atoms, 2 elements.
What is the difference in a compound and a molecule?
All compounds are molecules with different elements.
Is this a balanced chemical equation? How do you know?
2Na + Cl2 --> 2NaCl
Yes, because there are the same amount of atoms on both sides.
The center of the atom and made of protons and neutrons.
Nucleus