What is labeled with a question mark?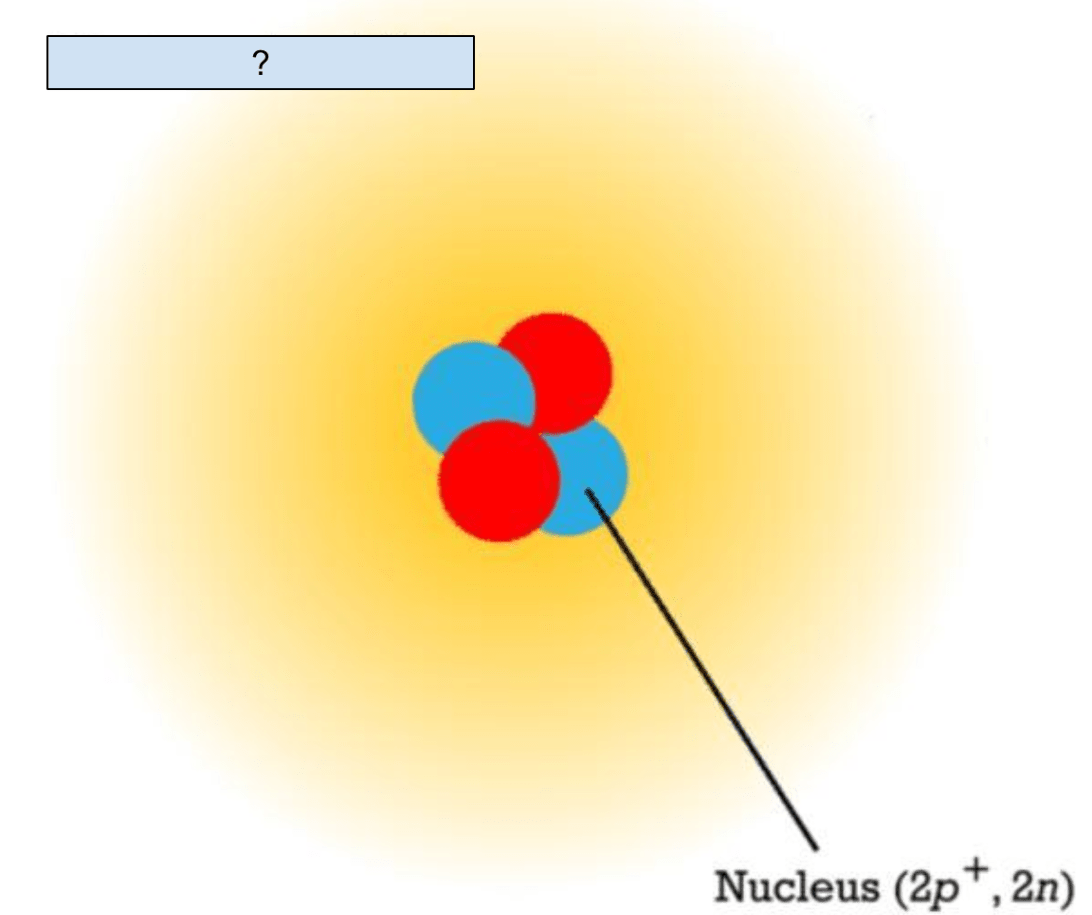
Electron Cloud
A positively charged subatomic particle
Proton
What are the columns called?
Groups or Families
What do we call the number at the top?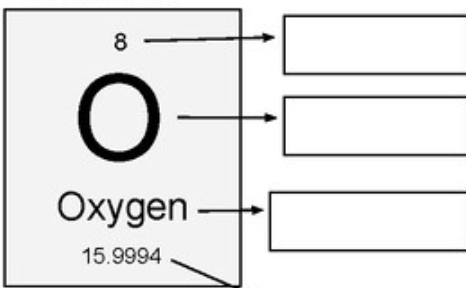
The atomic number
Rank from big to small:
protons, atoms, electrons
1.Atoms
2. Protons
3. Electrons
Where are the protons and neutrons located in a cell?
The nucleus
The number of protons determine the ________
element
There are metals, nonmetals, and ...
metalloids
The atomic mass is made up of these 2 subatomic particles
Protons & Neutrons
What Hoover sport does Mrs. N coach?
Girls Basketball
Who discovered / created the atomic model that looked like this: 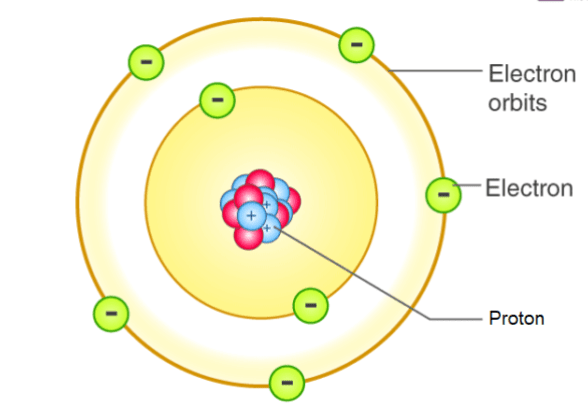
Niels Bohr
When electrons are gained or lost, these form
ions
What is the symbol for Helium?
He
Increases from left to right across the periodic table
atomic number
How do you spell Mrs. N's last name?
Niedzielski
In the early 1800s, John Dalton correctly recognized the relationship between atoms and elements: _______ are groups of ________ that are identical.
elements are groups of atoms that are identical.
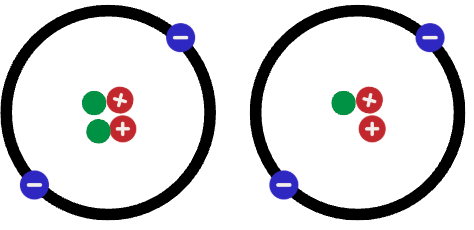
Isotopes
What information is shown on an element Key?
element name, symbol, atomic number, and atomic mass
How many neutrons are in 1 atom of Zinc?
35 neutrons
What was Mrs. N's last name before she got married?
Solorzano
What did Ernest Rutherford discover from his gold foil experiment?
atoms are mostly empty space with a tiny, dense, positively charged center called the nucleus
What forms when electrons are removed from an atom
Cation
levels of reactivity
If an element below Francium (Fr) was discovered, how many protons would it have?
119 protons
Which particles are the exact same element?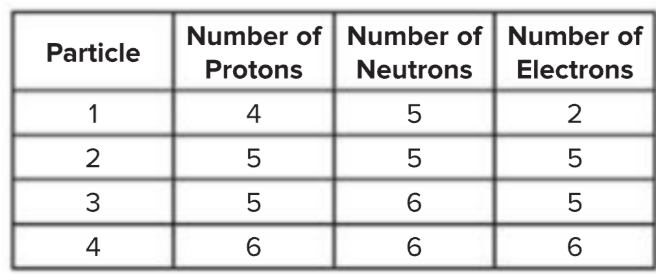
Particles 2 & 3