What particles are located in the center of an atom?
Protons and Nuetrons
How many elements are on the periodic table?
118
Cutting paper is an example of a chemical of physical change?
Physical Change
Does light have matter?
No. It does not have matter.
Who created the first model of an atom?
John Dalton
How many elements did the first periodic table have?
55 elements
To change the an atom of a specific element you MUST change the number of________________.
Protons
How many protons does Sodium have?
11 Protons
This type of change in state is water to a gas (when water is boiling).
Vaporization
What is matter?
Anything that has mass and takes up space.
Who created the first periodic table?
Dimitri Mendeleev
What is the Atomic Mass? (What makes up the Atomic mass).
Atomic Mass: The number of protons and neutrons in an atom
Draw an atom (include the major parts).
Label all the parts.
What type of element is silicon?
Metaloid
What type of change is "leaves turning brown, and falling off the tree"
Chemical because those are dead leaves
Density can be defined as......
The amount of stuff something has.
What did Ernest Thompson do in 1911?
(Hint: think of a thin sheet of gold)
He discovered atoms are mostly empty space.
What are four characteristics of a metal?
•Are silver-grey in color
•Are solids @ room temperature, except for Mercury
•Reflect light when polished (luster)
•Have high melting and boiling points
•Are attracted to a magnet
What is happening at lines A and B?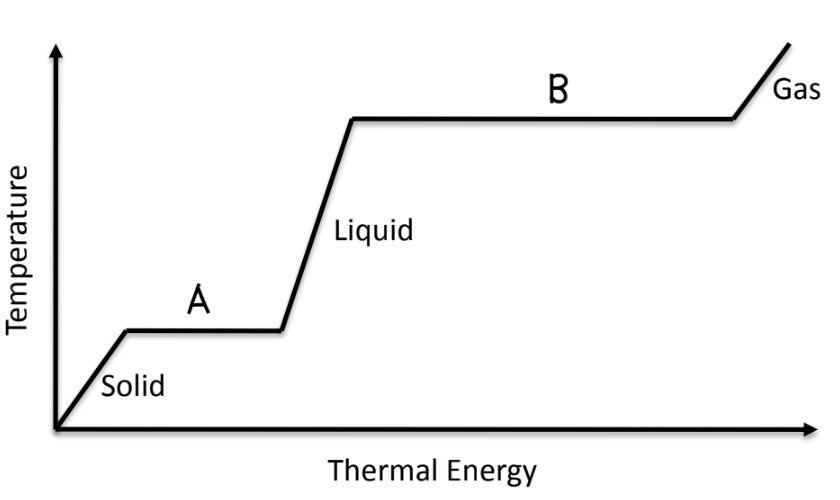
Matter is changing states
What are four characteristics of a metalloid?
•Are generally solids
•Can be shiny or dull (luster)
•May or may not be hammered flat (malleable)
•May or may not be drawn into wire (ductile)
•May or may not be brittle
•Conduct heat and electricity better than nonmetals, but not as well as metals
Give an example of a solid, liquid, gas, and plasma
Ice, Water, Water Vapor, The sun
What are the five signs of a chemical change?
Bubbles
Odor
Color Change
Change in temperature
Formation of a precipitate (Condensation)
Who discovered Electrons in 1897?
JJ. Thompson
If I have a atom with 24 protons, 24 neutrons, and 24 electrons what is the charge?
If an atom has a charge of -2, and 84 electrons. What element is that atom?
What rules are there about an element tile on the Perodic Table?
The atomic number cannot be larger than the atomic mass.
The chemical symbol can not have two capital letters in it.
Define Sublimation
Solid ---> Gas
What are four characteristics of a non metal?
Exist as solids, liquids, or gases @ room temperature
•Do not reflect light well (no luster)
•Are brittle
•Cannot be hammered flat (not malleable)
•Cannot be drawn into a wire (not ductile)
•Are soft and bend or break easily (low tensile strength)
Who discover Neutrons in 1932?
James Chadwick
If an atom has 5 electrons and a charge of -1. How many protons does it have?
4 Protons