100 Q: If an atom has an atomic number of 8, how many protons does it have?
8
APE-MAN Breakdown
A (Atomic number) = 8
P (Protons) = 8 (atomic number = number of protons)
E (Electrons) = 8 (neutral atom → same as protons)
M (Mass number) = ? (not given)
N (Neutrons) = ? (not given)
100 Q: True or False: Metals are good conductors of heat and electricity.
True
100 Q: Which subatomic particle has a positive charge?
Proton
(remember the P in Proton stands for Positive)
100 Q: What are the horizontal rows of the periodic table called?
Periods
(remember the periodic table is like a sentence and the periods go at the end (left and right) of the sentence.
100 Q: Which of the following determines the identity of an atom?
Protons
“P = identity” → Protons tell you who the atom is.
Electrons and neutrons can change (ions or isotopes), but the element stays the same as long as the protons stay the same.
200 Q: A neutral atom has 15 protons. How many electrons does it have?
A: 15
APE-MAN Breakdown:
A = 15
P = 15
E = 15 (neutral → electrons = protons)
M = ? (not given)
N = ? (not given)
200 Q: Which side of the periodic table are nonmetals found on?
Right side
200 Q: What is the charge of a neutron?
Neutral (0 charge)
(remember the Nue in Neutron is like the Nue in Neutral
200 Q: What are the vertical columns of the periodic table called?
A: Groups (or Families)
Remember that elements in groups have similar traits and behaviors like siblings in a family would (up and down)
300 Q: Assume this atom is neutral. Using your periodic table and the diagram shown, what element is this? 
Helium (He)
(based on the number of electrons(2) and APE-Man, we can figure out that the atomic number is 2)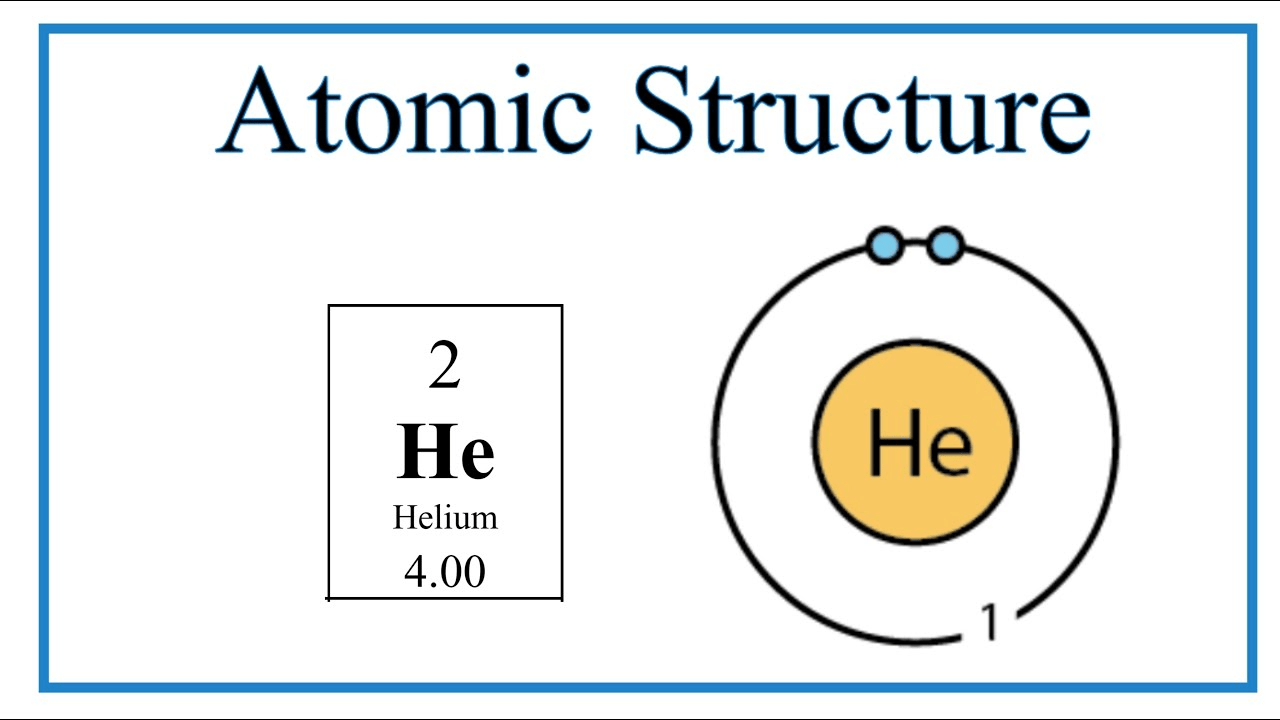
300 Q: If an atom has 6 protons and a mass number of 13, how many neutrons does it have?
A: 7
APE-MAN Breakdown:
A = 6 (atomic number = # protons)
P = 6 (given)
E = 6 (neutral atom)
M = 13 (given)
N = M − P = 13 − 6 = 7
300 Q: Name main defining property of metalloids.
Can act like metals and nonmetals (semiconductors)
300 Q: Which subatomic particle orbits around the nucleus?
Electron
(Electrons Exit” – electrons are outside, moving around the nucleus. )
300 Q: Which group is called the Noble Gases?
Group 18
(ALL the way to the right)
300 Q: Assume this atom is neutral. Using your periodic table and the diagram shown, what element is this? 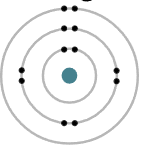
Magnesium (Mg)
(based on the number of electrons(12) and APE-Man, we can figure out that the atomic number is 12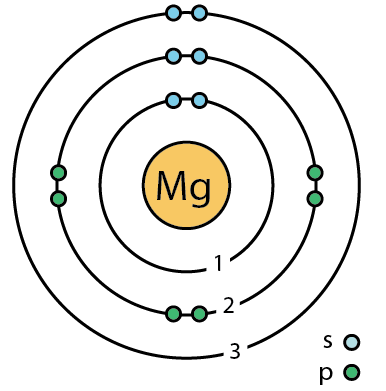 )
)
DAILY DOUBLE:
Daily Double Rules
You can wager any amount of your points (up to all of them).
Correct answer: you win your wager.
Wrong answer: you lose your wager.
If you have 0 points: you can still wager the question’s points, but your score can go negative.
💡 Tip: Bet wisely – it could double your points or drop you into the red!
400 Q: An atom has 9 protons, 10 neutrons, and 9 electrons. What is its atomic number and mass number?
Atomic number = 9
Mass number = 19
A (Atomic number) = number of protons = 9
P (Protons) = 9
E (Electrons) = 9 (neutral atom → electrons = protons)
M (Mass number) = P + N = 9 + 10 = 19
N (Neutrons) = 10
400 Q: Silicon (Si) is classified as which type of element?
Metalloid (Silicon is on the steps)
400 Q: What two particles are found in the nucleus?
Protons and neutrons
(“Protons & Neutrons Party in the Nucleus” – they’re the “guests” in the center.
“Electrons Exit” – electrons are outside, moving around the nucleus.)
400 Q: Sodium (Na) is in period 3. What does that tell us about its electron shells?
A: It has 3 electron shells
“Period = layers” → think of each row as adding another “layer” of electrons around the nucleus.
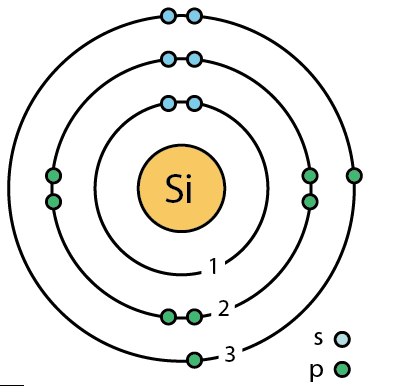 400 Q: How many valence electrons does Silicon (Si) have?
400 Q: How many valence electrons does Silicon (Si) have?
4 Valence electrons
(remember valance electrons are the electrons on the outer shell) 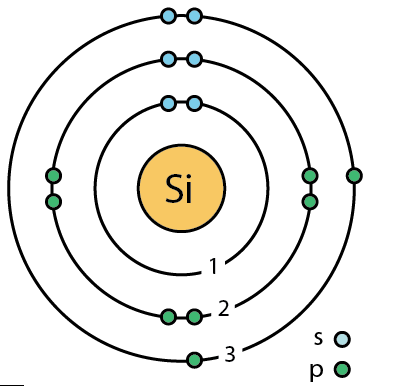
A neutral atom has 20 protons and 20 neutrons. What is its atomic number and mass number?
Atomic number = 20, Mass number = 40
APE-MAN Breakdown:
A = 20 (atomic number = # protons)
P = 20
E = 20 (neutral → electrons = protons)
M = P + N = 20 + 20 = 40
N = 20
500 Q: Which category of elements is brittle, dull, and poor conductors?
A: Nonmetals
500 Q: The mass of an atom comes mostly from which two particles?
Protons and neutrons
(Remember electrons is about 1/1836 the mass of a proton)
500 Q:
1. Identify the element in Group 17, Period 3 → ________
2. Identify the element in Group 18, Period 2 → ________
1.Group 17, Period 3 → Chlorine (Cl)
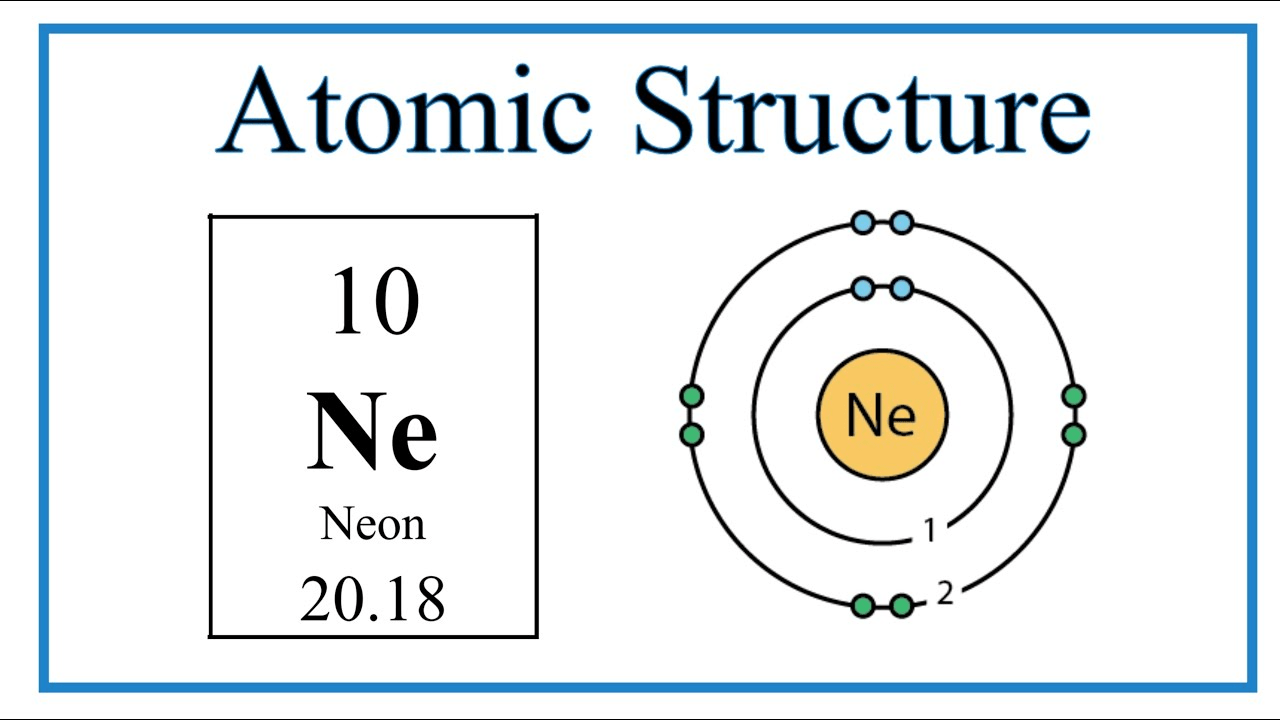
2. Group 18, Period 2 → Neon (Ne)
On the whiteboard: Draw this element in a bohr model
include: Proton, Neutrons and Electrons, as well as the electron shell(s)