Each vertical column on the periodic table is called a(n
What is group/family?
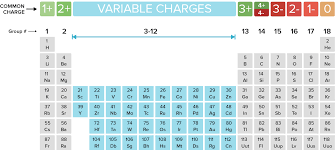
Name the following covalent compounds (1-4) and write the chemical formula for compounds 5-7. Remember covalent compounds include ONLY NON METALS that SHARE non paired valence electrons in their outer most shells. The prefix mono is only used in the second element of the compound. See the periodic table below that identifies valence electrons in each group/family (column).
- ClF3
- PCl5
- SO2
- N2O5
- CO
- carbon tetrafluoride
- selenium dichloride
- sulfur trioxide
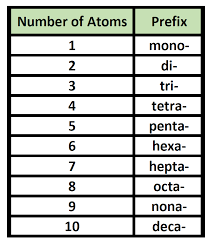

What are...?
- chlorine trifluoride
- phosphorus pentachloride
- sulfur dioxide
- dinitrogen pentoxide
- carbon monoxide
CF4
SeCl2
SO3
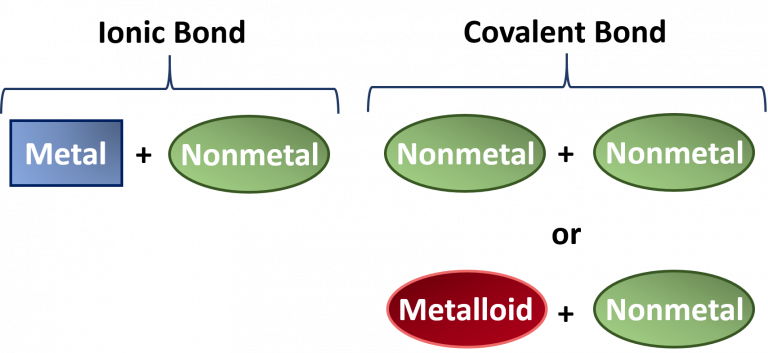
Covalent and Ionic bonds
1. Which compounds are covalent?
2. Which compounds are ionic?

What are....?
1. covalent compounds (non metals only): CH4, I2, C4, H2O,
2. ionic compounds (metals with non metals):BeCl2, KNO3, Fe2O3
The horizontal row on the periodic table is called a(n
What is period?
Which statement best describes the difference between the elements in group 2 and group 18 in the periodic table?
What is "2 are reactive metals, and 18 are unreactive gases"?
Name the following ionic compounds. REMEMBER, ionic compounds do not use the greek prefixes like covelent compounds. Ionic bonds form when a metal (in most cases) loses one or more electrons of their valence shell) and give them to a non metal. See the periodic table below to observe the trend of the unpaired valence electrons that are either lost (+) or gained (-).
- CaCl2
- AlF3
- KCl
- MgO
- Zinc bromide
- Aluminum oxide
- Calcium phosphide

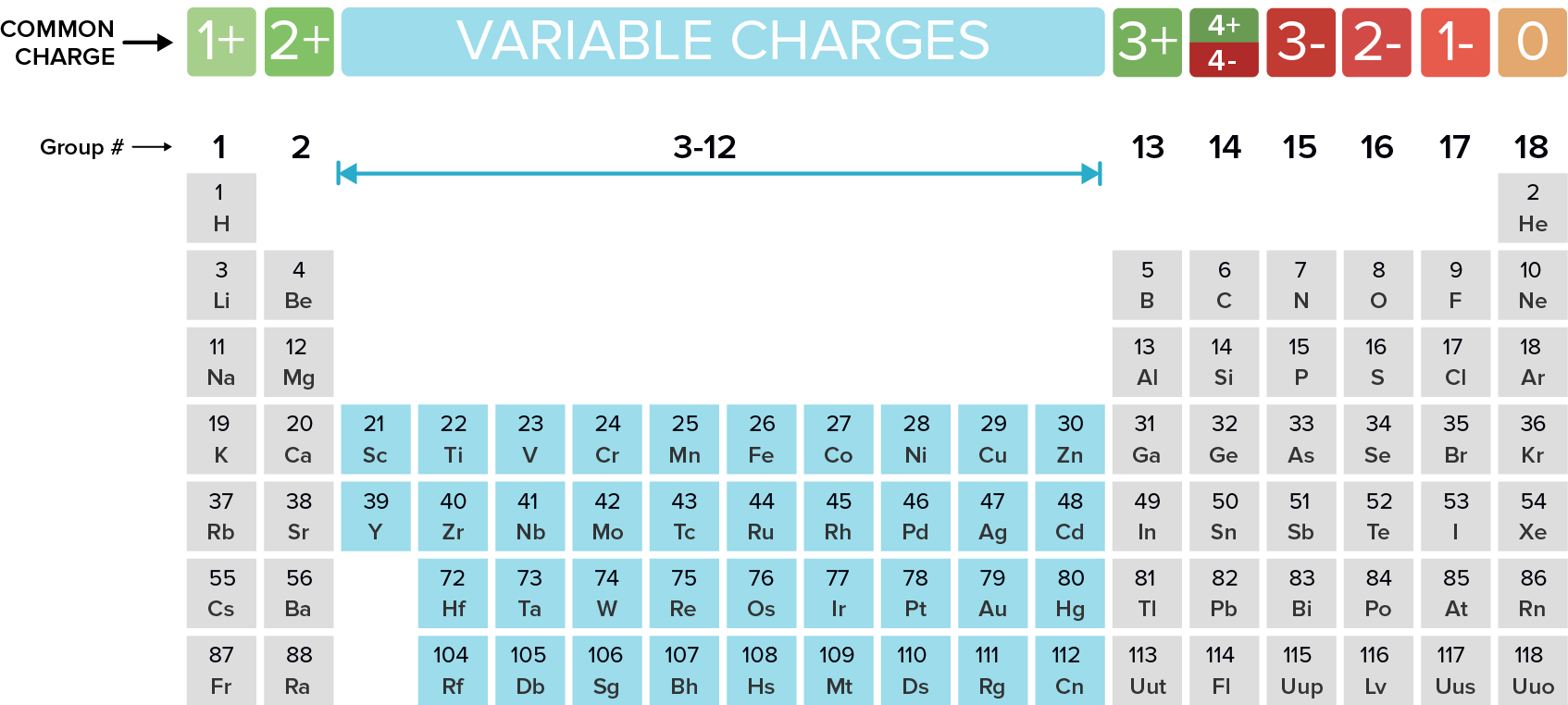
What are...
1. Calcium chloride
2. Aluminum fluoride
3. Potassium chloride
4. Magnesium oxide
5. ZnBr2
6. Al2O3
7. Ca3P2
Separate the physical and chemical properties of the elements from the list.
appearance
reactivity with other chemicals
texture
color
toxicity
odor
flammability
heat of combustion
oxidation state (number of electrons lost)
boiling point
density
solubility
oxidation state
chemical stability
What are...?
Physical properties:
appearance, texture, color, odor, melting point, boiling point, density, solubility
Chemical properties:
reactivity with other chemicals, toxicity, flammability, heat of combustion, oxidation state, chemical stability
What is in the center of an atom called?
What is the nucleus?
True/False Elements that are the most "metallic" (most metal like) are farthest to the right on the periodic table.
What is "False"? (Elements that are the most metallic are farthest to the left on the periodic table.)
How does the number of electrons in the outer level of the elements change as you move from left to right across the periodic table?

What is "the number of electrons increases"?
Barium is in Period 6 and Group 2. How many valence electrons does it have and how many electron shells does it have? (must answer in correct order)
What is "2" and "6"?
Transition metals
Which of the following is NOT a property of transition metals?
1. Their ions form colored solutions
2. They have a d electron shell
3. They react with water
What is
They react with water?
An atom of carbon with 6 protons, 6 electrons, and 6 neutrons would have a mass number of
What is 12?
True/False The atomic number (# of protons) is usually listed above the element's symbol/name and the atomic mass (# of protons+neutrons) is usually listed below the elements symbol/name.
What is "true"?
Why do elements in the same group share similar properties?

What is "they have the same number of electrons in their outer energy level"?
Ionization means how much energy is needed for an element to lose an electron, meaning to become a cation. Look at the 2 table below. Which element has the lowest ionization energy (meaning it loses its unpaired valence electrons without a lot of energy), and which element has the highest ionization energy (meaning, it loses its unpaired valence electrons but with a very high amount of energy).
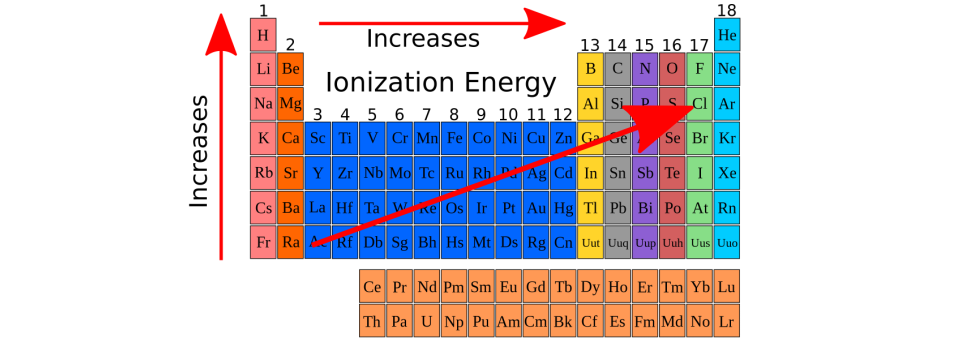
What is Francium has the lowest ionization energy, it becomes a cation very easily. Fluorine has the highest ionization energy, it is incredibly difficult for it to become a cation?
An atom has an atomic mass of 19 and an atomic number of 9. How many neutrons does the atom have? Name this element.
What is 10 and Fluorine?
How do the physical and chemical properties of the elements change?
What is "across each period"?
Sodium (Na) is a very reactive alkali metal and Chlorine (Cl) is a very reactive halogen nonmetal. When they combine, they react to form Sodium Chloride (NaCl) which is known as table salt. Which of the following best explains why sodium and chlorine so react so easily?
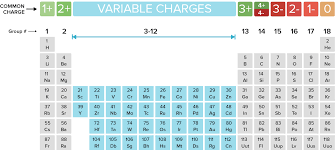
What is "alkali metals easily give away their single electron in the outer level and halogens only need one electron to fill their outer level"?
Balance the following chemical equations:
*** Remember to count the number of atoms in each compound before and after the chemical reaction and include the appropriate coefficients.
1 . ___ H2 + O2 ---> ___ H2O
2 . ___ S8 + ___O2 ---> ____ SO3
3. ____ HgO ---> ____ Hg + ____O2
4. ____ Zn + ____ HCl ---> ____ ZnCl2 + ___ H2
5. ___ Na + ___ H2O ---> ____ NaOH + ___ H2
What are (numbers in bold are coefficients)?
1 . 2 H2 + O2 ---> 2 H2O
2 . S8 + 12O2 ---> 8 SO3
3. 2 HgO ---> 2 Hg + O2
4. Zn + 2 HCl ---> ZnCl2 + H2
5. 2 Na + 2 H2O ---> 2 NaOH + H2
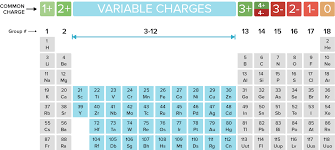
Oxidation is defined as the likelihood of an element to lose an electron as seen in the image below. For example, the elements in Group 1 lose 1 electron from the outermost shell.
Which elements always have an oxidation number of +1?
Which elements always have an oxidation number of -1?
What is the charge of an ion in Group 2?
1. What are the Alkaline metals?
2. What are the Halogens?
3. What is +2 (they lose 2 electrons in their outermost shell)?