What are the 3 parts of an atom?
What are protons, neutrons, and electrons?
What calcium will do with its electrons, and how many, to gain a full valence shell.
What is lose 2 electrons?
The hyphen notation of this isotope?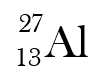
What is aluminum-27?
An arrangement of the elements by increasing atomic number and by changes in physical and chemical properties.
What is the periodic table?
The charge of protons?
What is positive?
What the rows of periods represent on the periodic table
What are how many electron shells an atom has?
What Oxygen will do with its electrons, and how many, to gain a full valence shell.
What is gain 2 electrons?
Hyphenated symbol for a magnesium atom that has 12 protons and 15 neutrons
What is Magnesium-27?
The symbol of an element with dots representing the number of electrons in the outer energy level
What is a Lewis Dot diagram?
The charge of electrons
What is negative?
What the group number 1A-8A represents
What is the number of valence electrons that an atom has?
Atoms that gain electrons become _______ charged and are called ________.
The atomic number of Helium-4
What is 2?
Where the neutron can be found in an atom
What is the nucleus of an atom?
The charge of neutrons
What is neutral?
What the atomic number represents
What is the amount of protons of an atom?
Atoms that lose electrons become ______ charged and are called _______.
What is positively, cations?
How you would write a hyphen notation for a carbon atom with a mass of 13
What is Carbon-13?
The most abundant isotope of oxygen?
oxygen-16
oxygen-17
oxygen-18
The charge of an atom
What is neutral?
What makes up the atomic mass of an element
What is the sum of the protons and neutrons of an atom?
Atoms that lose or gain electrons
What are ions?
Atoms of the same element that have same number of protons but different number of neutrons.
What is an isotope?
The Law of Conservation of Mass
What is matter cannot be created or destroyed?