Write its IUPAC name.
Common Name: Picric acid
IUPAC: 2,4,6-Trinitrophenol
I wear a crown of sulfur smoke,
My breath will make the strongest choke.
Not quite acid, not quite base,
I linger in an in-between place.
My name is whispered when reactions demand pain.
Oleum
(Fuming sulfuric acid – H₂SO₄ + SO₃)
Neopentyl chloride when treated with KOH dissolved in water undergoes this mechanism.
What is SN1 mechanism?
How many optically active isomers are possible for the compound 2,3-dihydroxybutanoic acid?
Maximum possible stereoisomers = 2ⁿ = 4
Of the five isomeric hexanes , the isomer which can give two monochlorinated compounds, is
2,3-dimethylbutane

Write its IUPAC name.
Common Name: Cinnamic Acid
IUPAC: 3-Phenylprop-2-enoic acid
I’m purple and nosy, I’ll ruin your day,
If your double bonds try to sneak past my way.
Alkenes I spot with a colorless clue—
So tell me, who am I? I’m watching you!
Baeyer’s Reagent 🟪
(KMnO₄ solution – turns colorless with alkenes!)
When cis-butene is treated with Bromine dissolved in CCl4 it will undergo this type of mechanism and produces a threo dl pair.
What is anti-addition?
Which of the following can show geometrical isomerism, optical isomerism, or both? Justify:
a) But-2-ene
b) Tartaric acid
c) 1,2-dichlorocyclopropane
d) Maleic and fumaric acid
c) 1,2-dichlorocyclopropane
An unknown hydrocarbon (A) with molecular formula C₇H₁₂ undergoes ozonolysis and gives a mixture of two compounds ethanal and 2-oxopropanal (methylglyoxal). Deduce the structure of compound A.
CH₃–CH=CH–CH₂–CH(CH₃)=CH₂
Name: 1-isopropyl-1,3-butadiene
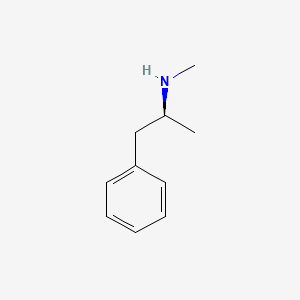
Write its IUPAC name. It's someone's favorite.
Common name: Methamphetamine
IUPAC: N-methyl-1-phenylpropan-2-amine
I’m made in dry ether, I’m flammable too,
Add me to carbon, I’ll build it for you.
But pour me in water? Oh no, that’s a fright—
I’ll fizz out in anger and vanish from sight!
Grignard Reagent 🔥
(RMgX – a drama queen when it sees water!)
Anisole upon treatment with HI will result in Phenol and Methyl iodide as a result of this pathway.
What is SN2 pathway?
Between cis-but-2-ene and trans-but-2-ene, which is more stable and why?
Trans-but-2-ene is more stable due to less steric hindrance
HE HE HE HEEEEEEEEEEE
😈 600 POINTS 😈
Free Points Enjoiiiiiiiii!

Write its IUPAC name. This is vital molecule for vision
Common Name: Vitamin A (Retino)
IUPAC: 3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetraen-1-ol
I love rings, especially with flair,
But I don’t bring roses—I bring nitro with care.
I’m mixed and I’m fuming, in acid I stew,
If your benzene touch me, it gets a no too!
Nitrating Reagent 💣
(Conc. HNO₃ + H₂SO₄ – brings explosive personality!)
A student treats compound A with chloroform and aqueous sodium hydroxide under heating conditions, and after acidification, obtains compound B, which gives a violet color with neutral FeCl₃ solution. This reaction follows this type of Mechanism
Wat is Electrophilic substitution on Benzene ring?
Vitamin A (retinal) exists in both 11-cis and all-trans forms. Which form is involved in vision, and how does its isomerization contribute to the visual process?
cis to trans
A compound X with molecular formula C₈H₁₄ undergoes ozonolysis to give two moles of the same aldehyde. Identify compound X.
4,5-dimethylhex-2-ene

Doesn't it look like penguin, anyways write its IUPAC name.
Common Name: Penguinone
IUPAC: 3,4,4,5-Tetramethylcyclohexa-2,5-dien-1-one
Call me the judge in a glassy court,
Where alcoholics line up to report.
I reward the bulk with clouds so fast,
But the slim must wait—they come in last.
Tell me, who tests spirits with a death stare?
Lucas Reagent
(Conc. HCl + ZnCl₂ – used to classify alcohols by reactivity.)
Secondary alkyl halides are borderline: they can undergo both SN1 and SN2 depending on conditions.
When sodium phenoxide is treated with isopropyl chloride (secondary alkyl halide), it will undergo this mechanism.
Wat is SN2 mechanism?
Citrus fruits like oranges and lemons predominantly contain d-limonene, while l-limonene is found in mint and pine. Despite being mirror images, they smell very different. Does smell have anything to do with optical activity?
Yes or No
Yes
Even though d-limonene and l-limonene have the same chemical formula, their three-dimensional shape differs due to chirality.
The olfactory receptors in our nose are also chiral and can distinguish between the two enantiomers.
Thus, d-limonene (from citrus) smells sweet and orange-like, while l-limonene (from mint or pine) smells sharp or turpentine-like.
This is C10H10 called [10] Annulene. Is this Molecule Aromatic or No?
Non Aromatic
It has 10 π-electrons → 4n + 2 = 10 → n = 2
It is cyclic, conjugated, and planar-looking in 2D drawings.
BUT
Due to steric hindrance and internal hydrogen crowding, [10]annulene cannot maintain planarity.
Lack of planarity breaks the condition for π-orbital overlap, making it non-aromatic despite satisfying Hückel’s Rule.