How many groups or vertical columns are on the Periodic Table?
18
What is the volume of Spongebob?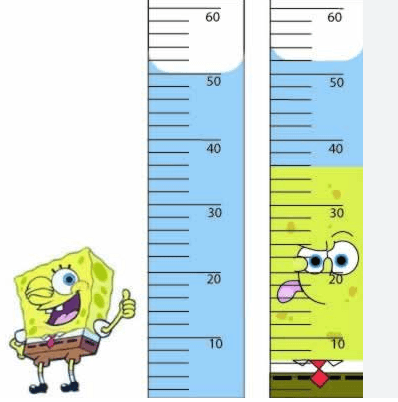
2
Which of the following represents an mixture?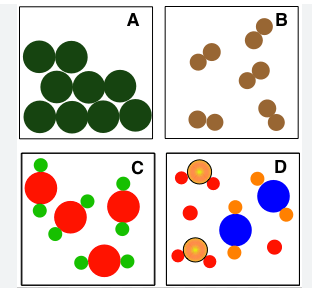
D
What type of reaction absorbs heat energy?
Endothermic
Which are not physical properties of metals?
Shiny, brittle, ductile, solids, poor conductors, soft, malleable.
brittle, poor conductors, soft
How does reactivity of elements change as you move from left to right along a period?
Reactivity decreases from left to right
To calculate Density you divide mass by what?
Volume
A pure substance made up of only one type of atom.
Element
This type of reaction releases heat.
Exothermic
Which properties are not properties on non metals?
Shiny, dull, brittle, ductile, poor conductors of heat, malleable, mostly gases at room temperature.
shiny, ductile, malleable
How many electron shells or energy levels do elements in Period 3 have?
3
The units for density can be expressed as grams per ________ or grams per ___________.
cm3, ml
Pure substance made up of more than one type of atom combined in a fixed ration.
compound
Exothermic
Which of the following are chemical properties?
Solubility, reactivity, acidity, luster, malleability, ductility.
reactivity, acidity
The electrons in the outer shell are called these type of electrons.
Valence
The density of an object that has a mass of 5g and a volume of 2 ml is?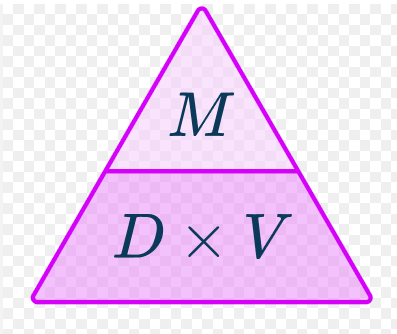
2.5 g/ml
A solution like salt water is this type of mixture in which the individual parts cannot be observed.
Homogeneous.
An acid reacting with a base is ___________ change
chemical
Property of metals that allows them to pulled into wires.
Ductility
Elements in Group 1 have one valence electron. Which group on the table do they tend to react with most readily?
Group 17
What is the volume of this object?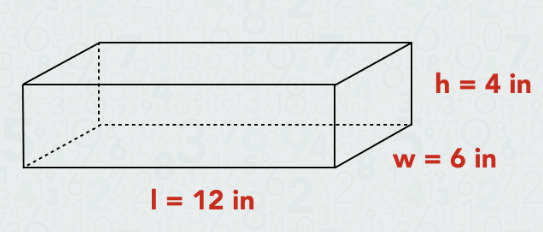
288 cu in
Which type of bond forms between a nonmetal and nonmetal?
Covalent
Which of the following are indicators of a chemical change?
Release of gas bubbles, increase in temperature, change in shape, formation of precipitate, release of light
all are correct except change in shape
Silicon is a very common element that is classified as a ___________ it allows electricity to flow when it is heated up?
Metalloid