Measuring Up
Measuring Up
What's the Matter?
Atoms & Elements
Atoms & their Chemistry
Covalent Compounds
Physical & Chemical Change
Stoichiometry
More Stoichiometry
This physical quantity is measured with liters
What is volume?
These particles are found in the nucleus of an atom.
What are protons and neutrons?
This is the mass of the average chlorine atom, in grams
What is 5.88x10-23g?
The following is an example of a p orbital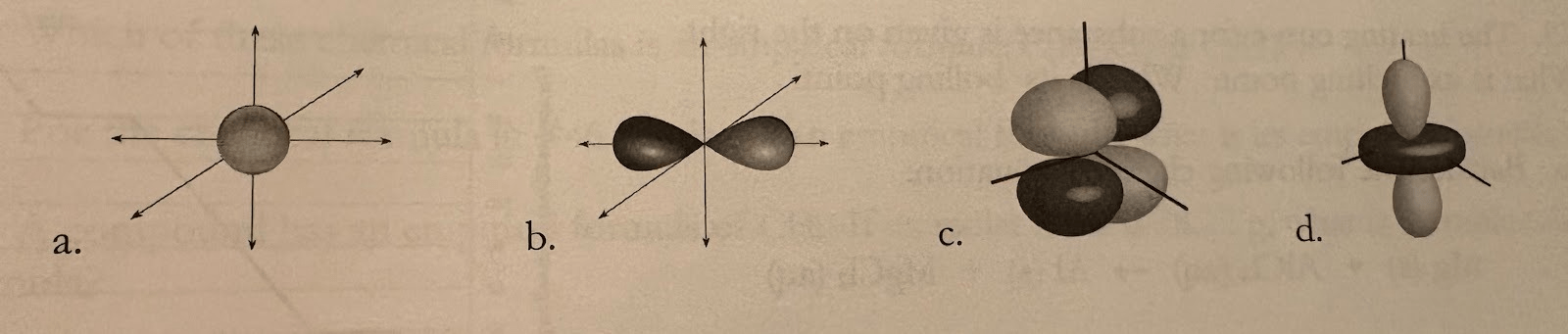
What is b?
Of the following, these molecules are likely to dissolve well in water:
CCl4
SiO2
NF3
What NF3?
Of the following, these are chemical changes
Water boils
Strontium chloride is decomposed into strontium and chlorine
- Ammonium chloride is dissolved in water
A single displacement reaction occurs
What are 2 & 4?
Hydrated cobalt (II) chloride has a chemical formula of CoCl2 . 6H2O.
a. This is the chemical formula of the anhydrous form of the same compound
b. This is how many water molecules there are for every one CoCl2
a. CoCl2
b. 6
This is the chemical formula of barium nitrate
What is Ba(NO3)2?
Convert a measurement of 16.2 kg into grams
In an experiment, a student burns 25.6 kg of wood. He collects all the products, finding that it produced 1.1 kg of ash, 48.2 kg of carbon dioxide, and 25.6 kg of water. This is how many kilograms of oxygen were consumed in the burning process.
What is 49.3g Oxygen?
A chemist is studying two isotopes of argon. The second has less mass than the first. This one has more neutrons.
What is the first one has more neutrons?
This is the full ground state electron configuration for sulfur
What is 1s2 2s2 2p6 3s2 3p4?
The following atoms are put in order of the electronegativity, from lowest to highest.
O Sr P Si
What is Sr, Si, P, O?
A substance turns from the solid phase to the liquid phase.
This substance gained / lost energy to do this.
What is gained energy?
This is the mass (in grams) of 169 moles of CO2
What is 7,440g CO2?
Of the following, this one has a chemical formulas that is an empirical formula. C6H12O6 / C2H2O
What is C2H2O?
An object has a mass of 0.112 kg. This is its mass in mg.
What is 112,000mg?
A chemist reacts 11.0 grams of carbon with 12.0 grams of oxygen to get 21.0 grams of a gas, along with some leftover carbon. In another experiment, she reacts 12.0 grams of carbon with 32.0 grams of oxygen to get 44.0 grams of a gas. This is the answer to are the two gases the same?
What is no they are not the same gas?
An element has 19 protons and 21 neutrons and this is its chemical symbol and mass number.
What is K 40?
This is the abbreviated ground state electron configuration for bromine (Br)
What is [Ar] 4s2 3d10 4p5?
For the molecule SiO2, this is it (a) its Lewis structure, (b) its shape, and (c) whether or not the molecule is polar.
What is?
a - Si double bonded to O2
b - linear
c - nonpolar
The heating curve for a substance
is shown
This is its melting point?
This is its boiling point?
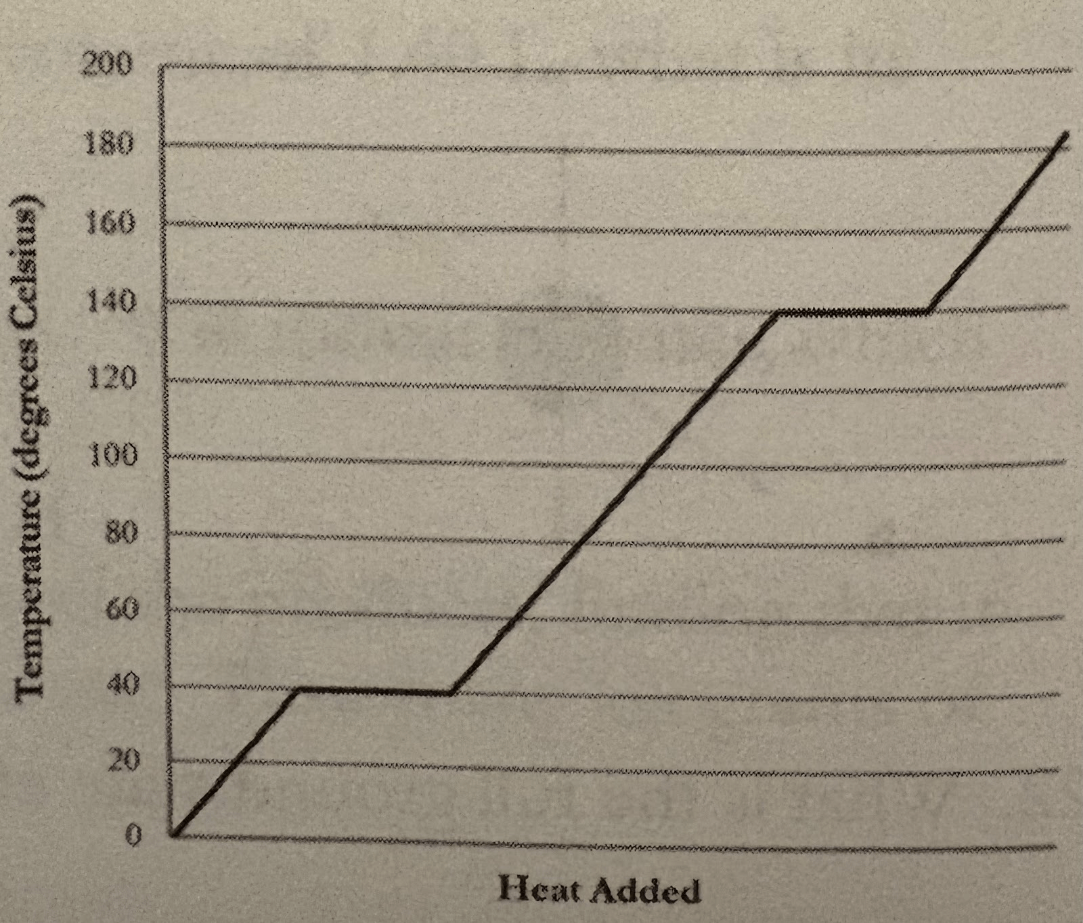
What is
Melting point - 40 C
Boiling point - 140 C
To go from mass to moles or moles to mass, don't forget to find this.
What is Molar Mass?
This is the empirical formula for the chemical formula of H6C12O6.
What is HC2O?
Copper has a density of 8.93 g/mL. If a copper statuette has a volume of 1.40 L, this is its mass.
What is 12,500g?
These two, of the following atoms, are isotopes
An atom with 20 protons, 20 electrons, and 21 neutrons
An atom with 21 protons, 20 electrons, and 21 neutrons
An atom with 22 protons, 22 electrons, and 22 neutrons
An atom with 20 protons, 20 electrons, and 20 neutrons
What are atoms A & D?
Between orange light and violet light, this light has a higher energy.
What is Violet light?
Of the following elements these ones should have very similar chemical behaviors
a. N b. C c. At d. Si e. Ag
What are b. C & d. Si?
For the molecule CCl4, this is it
(a) its Lewis structure, (b) its shape, and
(c) whether or not the molecule is polar.
What is ?
a - <one C in the middle with one Cl on each side
b - tetrahedral
c - nonpolar
Balance the following chemical equation:
Mg (s) + AlCl3 (aq) → Al (s) + MgCl2 (aq)
What is
3Mg (s) + 2AlCl3 (aq) -> 2Al (s) + 3MgCl2 (aq)?
A chemist makes manganese (II) chloride according to the following chemical equation:
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
He runs the reaction and finds that in the end, lots of MnCl2 is made, and the only other chemicals present are water, chlorine gas, and HCl. This was the limiting reactant.
What is MnO2?
A compound has an empirical formula of CH. If its molar mass is 78.12 grams, this is its molecular formula
What is C6H6?
A piece of plastic has a mass of 490 kg and a volume of 350 liters. Will it float in water, which has a density of 1.0 g/mL?
What is no, it will not float?
This subatomic particle has no charge
What is the neutron?
This is the most abundant isotope of calcium
What is Ca 40?
This is the chemical formula and name of the compound formed by magnesium and phosphorus
For the molecule NF3, this is its (a) its Lewis structure, (b) its shape, and (c) whether or not the molecule is polar.
What is?
a - N with three F and an unshared electron pair
b - pyramidal
c - polar
This is the balanced chemical equation for the decomposition of H3PO4.
What is?
2H3PO4 -> 3H2 + 2P + 4O2
The combustion of pentane (C5H12) occurs according to this chemical equation:
C5H12 (g) + 8O2 (g) → 5CO2 (g) + 6H2O (g)
If 112 moles of C5H12 are burned in excess oxygen, this is how many moles of CO2 are formed.
What is 5.60x10^2 moles of CO2?
A compound is 19.4% calcium, 34.3% chlorine, and 46.3% oxygen. This is its empirical formula
What is CaCl2O6?