How many electrons can the p sublevel hold?
6 electrons
An ionic bond is formed between a ___________ and a ___________. (metal, non-metal, metalloid)
Bonus: How to electrons play a role in an ionic bond?
metal & non-metal
Bonus: Electrons are transferred from the metal to the non-metal. The opposite charges hold the bond together by attractive forces.
What is the empirical and molecular formula of C12H24O12?
M.F. - C12H24O12
E.F. - CH2O
Label the parts of the chemical equation:
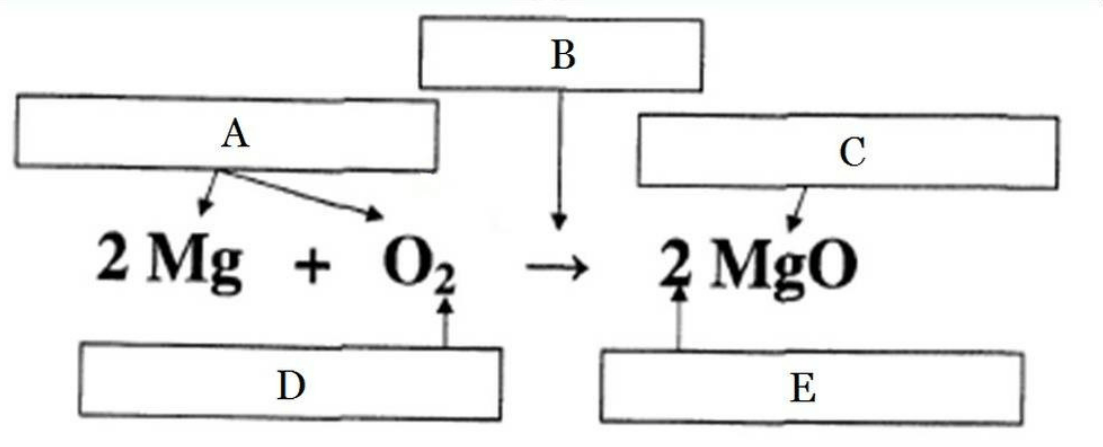
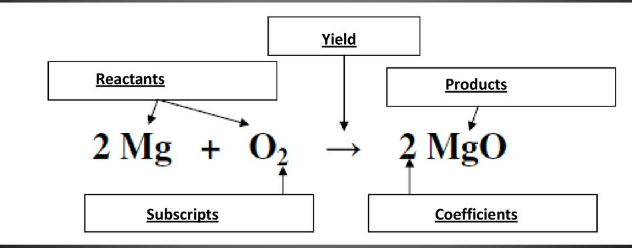
What will happen to a reaction once the limiting reactant has been used up?
What are the 4 types of REDOX reactions?
Synthesis, Decomposition, Single Replacement, Combustion
Which element has the ground state electron configuration of 1s2 2s2 2p6 3s2 3p5?
Bonus: Draw the lewis dot structure for the element
Chlorine
Bonus:
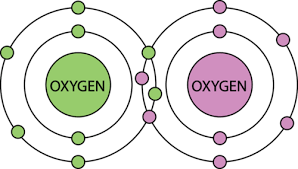
What type of bond is shown below? (ionic/covalent)
What is the name for FeBr3?
(included roman numerals if necessary)
Iron (III) Bromide
When energy or heat travels into the surroundings, making them warmer, this is known as an _____________ process. (endo/exo)
Exothermic.
What is the law of conservation of mass and how does it relate to chemical reactions?
Mass is neither created nor destroyed in chemical reactions.
*Note how the number of atoms of each kind of element before and after the reaction stays the same.
This means the mass stays the same as well.
= mass is conserved
The particle diagram shown represents which type of redox reaction?
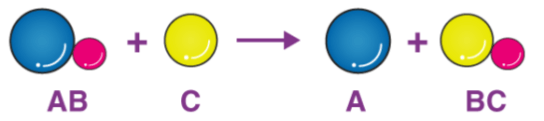
Single Replacement
Which wave is more harmful and why, Ultraviolet (UV) or visible light?
UV light is more harmful than visible light because they have shorter wavelengths = high frequency= high energy= more harmful
Draw the Lewis Dot Structure for NaCl.
What is the correct name for P2S5?
Diphosphorous pentasulfide
The PE diagram shown represents an _______________ (endo/exo) reaction.

Endothermic
Why do the reactants weigh more than the products in the image below?
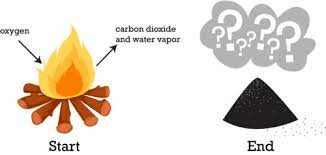
Gases were released into the surroundings. Mass is always conserved.
Which single replacement reaction (if any) will not occur and how do you know?
All of the reactions will occur because the single metal can replace the bonded metal because it is higher on the activity series.
The orbital diagram shown represents which element?
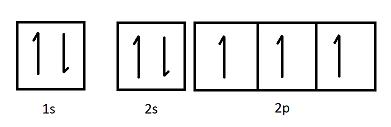
Nitrogen.
Draw the Lewis Dot Structure for F2.
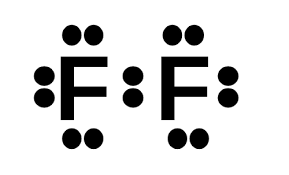
What is the formula for ammonium chromate?
(NH4)2CrO4
Balance the following synthesis reaction:
____ Mg + ____ O2 -> ____ MgO
2, 1, 2
For the following reaction, if 2 moles of propane react with excess oxygen, how many moles of CO2 and H2O will be produced?
x2 propane is being used so products will also x2.
6 moles of CO2 and 8 moles H2O will be produced
Loss of electrons is known as ____________ (oxidation/reduction).
oxidation
What is the shape of water (H2O)?

Bent
Which sample is an electrolyte?
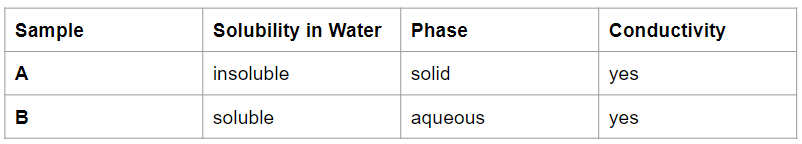
Only Sample B is an electrolyte because it is soluble in water (aqueous) and can conduct electricity.
What percentage of Carbon Dioxide is Carbon?
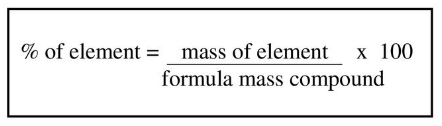
ΔH = -890.3 kJ
does this value represent an endothermic or exothermic reaction?
Exothermic (negative ΔH)
How many liters are in 3.00 moles of O2 at STP?
The half reaction shown represent which? (oxidation/reduction)
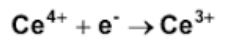
Reduction