Theobromine (C7H8N4O2) is a molecule found in chocolate that is toxic to dogs. The amount a dog can eat depends on its weight, for my 24 lb corgi 7.75 X 10-4 moles or more of theobromine can be dangerous. If I used 2 bags of chocolate chips in the cookie dough, with each bag weighing around 340.19 g and containing 0.1552% theobromine by weight, could my dog eat the whole batch of cookies? Answer must include the theobromine content.
No (0.00586 moles)
What is the name and three letter abbreviation of the following amino acid

Asparagine (ASN)
What are the three types of inhibitors?
Competitive, uncompetitive, mixed
Enzymes affect kinetics not thermodynamics
What are the four mechanisms you need to have memorized?
Carbonic, anhydrase, RNase, chymotrypsin, lysozyme
How many chiral centers does this molecule have?
5
Draw tryptophan

What does each type of inhibitor interact with?
Competitive: active site
Uncompetitive: ES complex
Mixed: both
Graph a first order reaction with and without an enzyme? (hint: rate on y-axis, concentration on x-axis)
linear relationship for without enzyme, logarithmic with
What is the catalytic triad in chymotrypsin? Draw out nucleophile generation
His57, Ser195, Asp102.

Hg2Br2 is a slightly soluble solid that dissociates according to the reaction:
If the concentration of Hg22+ in a saturated solution of Hg2Br2 at 25°C is found to be 2.44 × 10−8 M. What is the value of Ksp for this salt?
5.81 X 10^-23
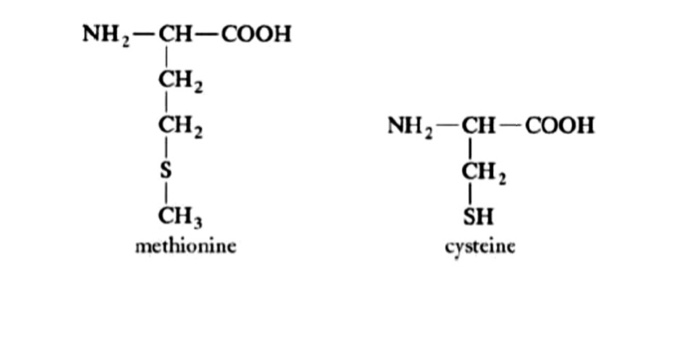
Draw the two sulfur-containing amino acids
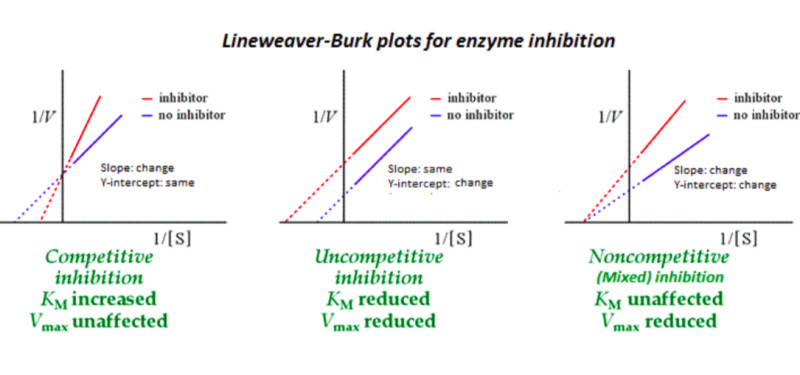
How does each inhibitor impact Vmax and Km?
Competitive: no impact on Vmax, Km increases
Uncompetitive: both decrease
Mixed: Vmax decreases, Km depends
How does an increase in Kd affect substrate affinity?
Lower affinity
What are the classifications for the two intermediates in the proposed mechanisms for lysozyme?
Covalent and carbocation
You are excited to start working in your garden and you want to sow your lettuce seed today, but in order to do so the temperature has to be below 26.67 °C or the seed will go dormant. Unfortunately, you woke up late and missed the beginning of the weather forecast. All you heard was that the dew point is 21.24 °C and the relative humidity is 75%. What is the temperature today?
Given:
299.178 K or 26.03 ℃
How many carbons and nitrogens are in the side chains of lysine and arginine?
4 carbons in both. 1 nitrogen in lysine, 3 in arginine
Draw the Lineweaver-Burk plots depicting the effects of each type of inhibitor
When [ET]=4 nanomolar and Vmax=1.6 micromolar/seconds, what does kcat equal?
400 1/sec
Draw out the mechanism for the generation of the catalyst in a carbonic anhydrase reaction
See physical copy
Calculate ΔS (for the system) when the state of 2.00 mol diatomic perfect gas molecules, for which Cp,m = 7/2 R, is changed from 25 °C and 1.50 atm to 135 °C and 7.00 atm.
-2.12 J/K*mol
What are the polar neutral amino acids?
Tyrosine, Serine, Threonine, Asparagine, Glutamine, Cysteine.
Write out the relationship between alpha and Ki
alpha= 1+ (concentration of inhibitor/Ki)
At what substrate concentration would an enzyme with a Kcat of 30.0 1/sec and a Km of 0.0050 M operate at 1/4 of its maximum rate?
1.7x 10^-3 M
Draw out the mechanism for the hydrolysis of a phosphodiester bond via RNase
