These structures make up matter and cannot be broken down further
Elements
This is used to balance elements and form compounds
A bond
These elements make up water
Oxygen and Hydrogen
Chemical reactions need energy to reach their
Activation energy
Organic compounds are
Carbon based
depending on the energy level, matter can have these three properties
solid, liquid, gas
This type of bond is very strong and shares electrons equally between elements 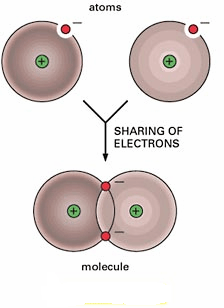
Covalent
This is the chemical make up of water
H2O
This compound in the biological energy source
ATP (adenosine Triphosphate)
Large compounds are known as
Macromolecules
These are the 4 major elements for life
Carbon, Hydrogen, Oxygen, Nitrogen
The bond causes the compound to have slightly charged potions
Polar Covalent Bond
These bonds are constantly breaking and reforming between water molecules
Hydrogen Bonds
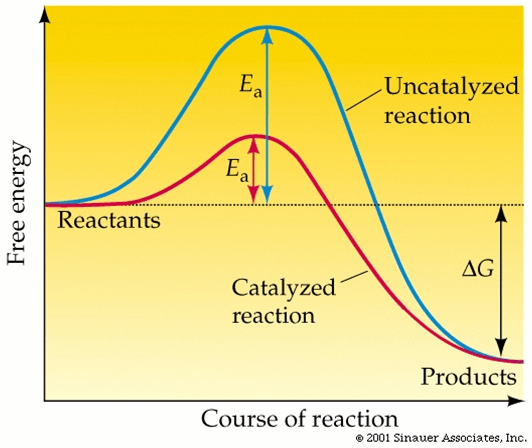
This compound is used to lower the activation energy of a reaction
Enzyme/Catalyst
The general term for the building blocks of macromolecules
monomers
These structures of an atom are exchanged to form bonds between elements
Electrons
Hydrogen bonds work by using _________________ to hold molecules together
attractive forces
This bond give water its major property allowing it to dissolve polar compounds
Polar Covalent
In ATP this portion is broken to release energy
the third phosphate group / the bond between the second and third phosphate
This is the portion of a compound that influences how that compound will react
Functional groups
living and non-living matter are made up of
the same elements
This reaction is used to build the bond between monomers
Condensation/Dehydration
The bonds found in water allow for these three properties of water
Adhesion
Cohesion
Capillarity
* Will accept: Polar
Draw the diagram representing the a reaction with and without an enzyme. Label the reactants, the activation energy, each reaction and products.
This is the reaction used to break down macromolecule carbon compounds
Hydrolysis