Name the 3 domains with their respective kingdoms
Bacteria: Eubacteria
Archaea: Archaebacteria
Eukarya: Plantae, Fungi, Animalia and Protista
What are the 4 most abundant elements that make up the human body?
O, C, H, N,
What is the name of an atom that is charged?
An ion
What are the 4 types of chemical bonds?
Covalent bonds, ionic bonds, hydrogen bonds and Van der Waals interactions
What is the difference between adhesion and cohesion?
Cohesion is the property of water sticking to another water molecule, while adhesion is the property of water sticking to a different molecule?
Explain the pH scale
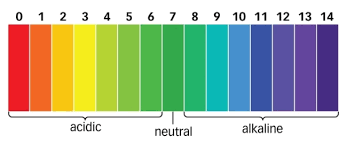
Answers may vary
What is the smallest unit of matter?
An atom
The three subatomic particles that comprise an atom are ______, _______, and an _______.
Proton, neutron, electron
In any hydrogen atom, which of the subatomic particles will always be the same?
Protons
What type of chemical bonding is present in methane (CH4)?
Nonpolar covalent
What does it mean to have a high specific heat capacity?
You require a lot of heat to raise the temperature.
7.4
Name the 7 properties of life
Order, evolutionary adaption, growth and development, regulation, energy processing, response to the environment, and reproduction.
The mass of an atom is located in the ______, while most of the volume is located in the _______.
Nucleus
Electron Cloud
What is the relationship between protons and electrons in an atom containing a positive charge?
P>E
A negatively charged atom is called a _____.
Anion
What happens to the density and volume of water when the temperature is >4oC? What happens to the density and volume of water when the temperature is <4oC?
Density gets lower while volume gets higher.
Define the following terms:
Solvent, solute and solution
Solvent: the dissolving agent of a solution
Solute: the substance that is dissolved
Solution: a liquid that is a completely homogeneous
mixture of substances
What is DNA?
What is a gene?
Genetic material
Units of inheritance (book full of DNA)
Find the charge, mass number and atomic mass of this hydrogen ion
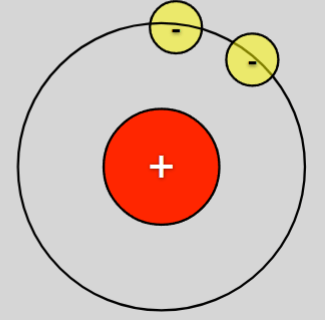
-1, 1, 1
How many valence electrons does a S atom have?
6
The product of ionic bonding is called a _____.
salt
Name the 4 emergent properties of water.
Cohesion/Adhesion
High Specific Heat capacity
Expands upon freezing
Dissolves and dissociates
What is [H+] of a solution with a pH of 3?
10-3 M
Difference between a eukaryotic cell and a prokaryotic cell
eukaryotic cell is bigger and has a nucleus while a prokaryotic cell is smaller and lacks a nucleus
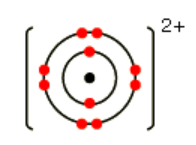
Propose a shell diagram for a Mg2+ ion.
Indicate how many of each subatomic particle is present in the following ion:
32P-
Protons: 15
Neutrons: 17
Electrons: 16
A hydrogen bond only occurs between?
H-F
H-O
H-N
What does it mean to be an aqueous solution?
The solvent is water.
Acids _____ (attach/release) H+ in a solution, while bases ______ (attach/release) H+ in a solution.
Release
Attach