This step of the scientific method is usually proposed as an "if...then..." statement.
Prediction
What is the smallest unit of matter?
An atom
What is the name of an atom that is charged?
An ion
What are the three types of chemical bonds?
Polar covalent
Nonpolar covalent
Ionic
What is the difference between adhesion and cohesion?
Cohesion is the property of water sticking to another water molecule, while adhesion is the property of water sticking to a different molecule?
What is the formula used to find the pH when given the [H+]?
pH = -log[H+]
The following claim is an example of what part of the scientific method:
Blue light increases plant growth.
Hypothesis
The three subatomic particles that comprise an atom are ______, _______, and an _______.
Proton, neutron, electron
In any hydrogen atom, which of the subatomic particles will always be the same?
Protons
What type of chemical bonding is present in methane (CH4)?
Nonpolar covalent
What does it mean to have a high specific heat capacity?
You require a lot of heat to raise the temperature.
7.4
The ________ __________ is a method employed by posing multiple hypothesis then using deduction to eliminate poor explanations.
Hypothetico-deductive method
The mass of an atom is located in the ______, while most of the volume is located in the _______.
Nucleus
Electron Cloud
What is the relationship between protons and electrons in an atom containing a positive charge?
P>E
A negatively charged atom is called a _____.
Anion
What happens to the density and volume of water when the temperature is >4oC? What happens to the density and volume of water when the temperature is <4oC?
Density gets lower while volume gets higher.
What is the name of the buffer used to control pH in the human body, as well as it's ion?
Carbonic Acid
Bicarbonate Ion
Four groups of rats are first massed then fed identical diets, except for the amount of vitamin A they receive. Each group gets an increasing amount of vitamin A. After 3 weeks, the rats' masses are measured and compared.
Identify the independent variable and dependent variable.
IV - [Vitamin A]
DV - mass of the rats
What are the ion(s) important in bone formation?
Ca2+
Why is the atomic mass for each element on the periodic table NOT a whole number?
It is an average of the mass of all existing isotopes and their relative abundance in nature.
The product of ionic bonding is called a _____. These are attracted to form a ______.
salt
lattice
Name the 4 emergent properties of water.
Cohesion/Adhesion
High Specific Heat capacity
Expands upon freezing
Dissolves and dissociates
What is [H+] of a solution with a pH of 3?
10-3 M
What relationship does a line of best fit with a downward slope demonstrate?
Inverse Correlation
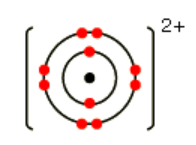
Propose a shell diagram for a Mg2+ ion.
How many valence electrons does a S atom have?
6
In a molecule of water, the ____ atom(s) will have a partial positive charge, while the _____ atom(s) will have a partial negative charge.
H
O
How many grams of NaCl does 500 mL of a 0.25 M solution contain?
What makes an acid such as HCl as "strong acid"?
It completely dissociates into it's ions: H+ and Cl- when put in an aqueous solution.
Which graph will have an r2 value closer to 1?
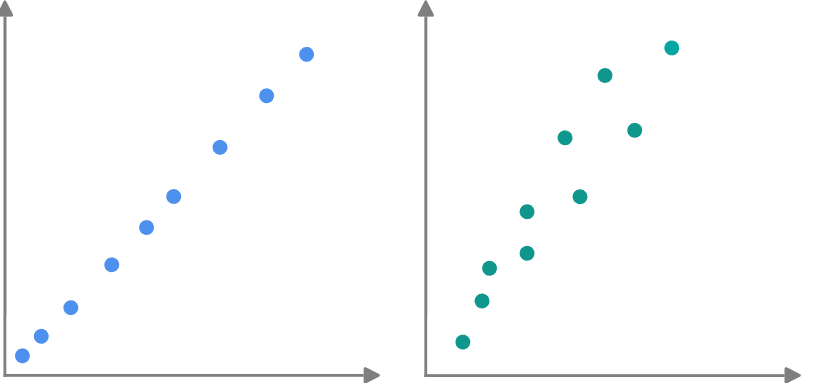
Left
Indicate how many of each subatomic particle is present in the following ion:
32P1-
Protons: 15
Neutrons: 17
Electrons: 16
What is the name of this ion:
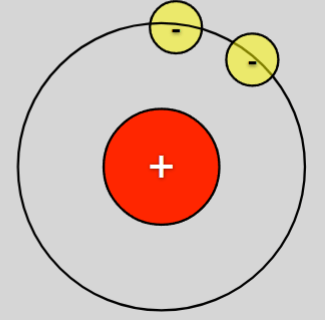
Hydride ion
This type of bonding is characterized by the unequal sharing of electrons. What is an example of a molecule containing this type of bonding?
Polar covalent. Water or ammonia.
If I have 30g of KBr, what value do I need in order to find out how many moles of KBr I have?
The molar mass of KBr.
What kind of base is ammonia considered? What ion is created after association with an H+?
Bronsted-Lowry base.
Ammonium Ion = NH4+
What is the experimental variable in the following graph?
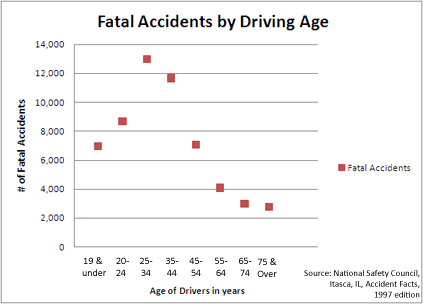
Age of drivers
What are the ion(s) important in oxygen transport? Muscle contractions?
Fe2+
Na+, K+, Ca2+
What are the 6 most abundant elements that make up the human body?
O, C, H, N, Ca, and P.
The physical bond involved in the dissociating of salts is called _______ ________.
Hydrogen Bonding
What does it mean to be an aqueous solution?
The solvent is water.
Acids _____ (attach/release) H+ in a solution, while bases ______ (attach/release) H+ in a solution.
Release
Attach