True or false - nonliving things may have some of the properties of life
TRUE
To be alive you have to have ALL 8 properties of life but not living things often have some (Example cars can move and computers can respond to stimulus but neither are alive)
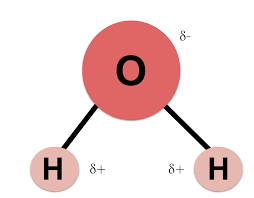
Draw a water molecule (including charges)
Enzymes are an example of which Macromolecule?
Proteins
How do animals get access to the Nitrogen they need to build molecules?
Eating/Feeding
This is the name of the monomer of proteins
Amino Acids
This property of life allows organisms to maintain consistent internal conditions
Homeostasis
What is the term for the phenomenon where water molecules are attracted to different substances?
Adhesion
The image below in the monomer for which macromolecule?

Nucleic Acids
This is the process that helps to remove Carbon Dioxide from the atmosphere
Photosynthesis
Cellulose and Starch are examples of this macromolecule
Carbohydrates
Define the difference between biotic and abiotic factors and give an example of each.
Biotic factors are alive or were once alive ( like an animal or a dead log) while abiotic factors have never been alive (like water or heat from the sun).
Which property of water is primarily responsible for its ability to dissolve many ionic and polar substances?
Universal Solvent
Which biomolecule is used for immediate energy by cells?
Carbohydrates
This organism helps fix nitrogen into a form usable by plants
Bacteria
These are the driving forces of the water cycle
Heat from the sun and gravity
Give an example of a response to stimuli:
Sweating (response to heat)
Shivering (response to cold)
Plants growing towards sunlight
This chemical reaction uses water to break a polymer into its monomer subunits?
Hydrolysis
Give an example of a carbohydrate molecule:
Cellulose, Chitin, Glucose, Fructose, Starch, Glycogen
Parking lots and sidewalks paving over the ground increase water in this phase of the water cycle.
Surface Runoff
A researcher claims that the chemical properties of water molecules are partly responsible for the movement of water between abiotic and biotic systems in the hydrologic (water) cycle. Which property helps water cycle through biotic and abiotic systems?
Universal solvent:
The polarity of water molecules allow water to dissolve organic and inorganic nutrients required by living organisms.
Describe the general relationship between abiotic and biotic factors.
- Changes in abiotic factors can lead to significant shifts in biotic populations.
- Biotic factors depend on Abiotic factors for survival
- Elements cycle between biotic and abiotic factors
Draw two water molecules with their partial charges AND a dashed line to represent a hydrogen bond between them.

Give the full names of the two primary examples of nucleic acids
DNA: Deoxyribose nucleic acid
RNA: Ribose nucleic acid
Explain why plants and animals can't use the Nitrogen found in the atmosphere?
N2 has a triple bond which makes it very difficult to break.
(This is why plants rely on nitrogen fixation to convert the N2 into NH3 and other usable forms)
Give an example of a negative impact humans have on nutrient cycles:
• Human activity/impact
• The cycle it effects
• HOW it impacts that cycle.
The water cycle can be negatively impacted by pollution in the air or bodies of water which water molecules then stick to (through adhesion) and carries the pollution through the cycle often depositing pollutants in other areas.
The nitrogen cycle can be impacted by humans over using fertilizers which drain into the water creating algae blooms that disrupt the balance of aquatic ecosystems.
Carbon Cycle is impacted by the burning of fossil fuels which disrupt the balance of the carbon cycle by putting too much CO2 into the atmosphere and influencing climate change.