Yes, slope
Describe secondary structure
-local spacial arrangement of backbone
-R group does not directly participate
What is the difference between the size and strength of hydrogen bonds vs. covalent bonds?
H- bonds are larger and weaker than covalent bonds
List 3 functions of lipids
-fat storage, cushioning, temperature regulation, hormones, chemical messengers, chaperones
pH = pKₐ + log([A⁻]/[HA])
You can find pH, pKa, concentration of acid and base
What are the negative and positive allosteric modulators?
Positive: O2
Negative: H+, CO2, BPG
What amino acid is this and what pH is this form most likely in?
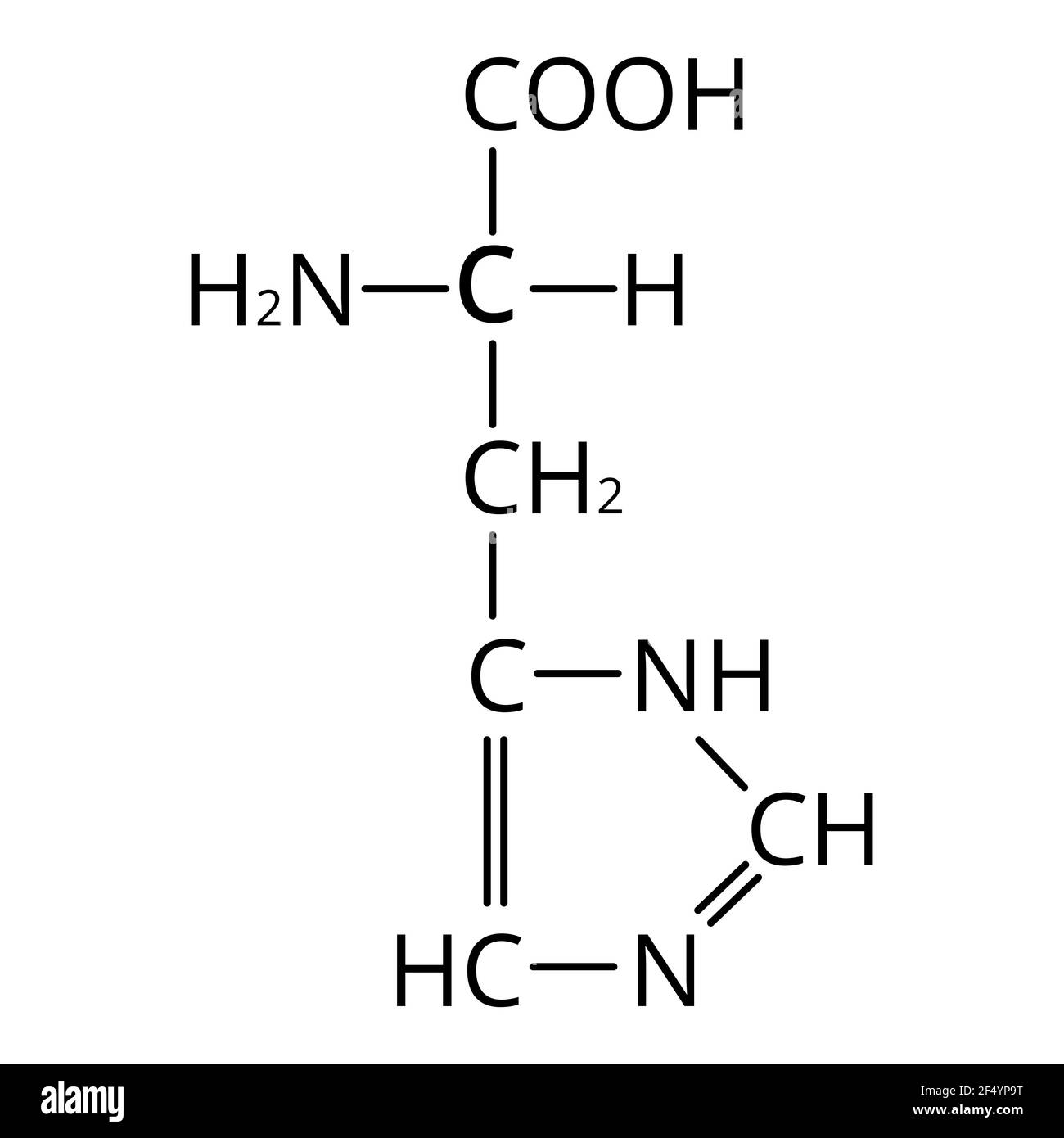
Histidine
pH 7
Is water a hydrogen donor or acceptor?
Both: oxygen can accept hydrogens and the hydrogens will be willing to donate their H to form a bond
Difference between saturated and unsaturated fatty acids
Saturated fatty acids do not have double bond kinks, which means it is more solid at room temperature than unsaturated.
Explain Van der Waal Interactions
Happens when 2 uncharged atoms are very close; There is a weak attraction that creates a transient dipole which induces an opposite dipole in the nearby atom.
Differences between R and T state
R: relaxed, oxy Hb, less salt bridges, high affinity, decreased BPG, decreased pKa, increased O2 binding
T: Tense, deoxy Hb, more salt bridges, low affinity, increased BPG, increased pKa, decreased O2 binding
What secondary structure does several gycines stabilize and destabilize? explain? (2 parts to the question)
Stabilize: Beta turns because it has a small R group
Destabilize: Alpha Helix because glycine's R group is H and is too small to prevent H2O from H- bonding with the backbone
How do you minimize order of water interactions?
Cluster the hydrophobic parts: Nonpolar regions cluster together to have the smallest surface area exposed to the aqueous environment.
How does and what forms stable vesicles?
Glycerophospholipids form stable vesicles because the structure of this lipid forms cylindrical shape which then forms a bilayer that spontaneously folds onto each other => stable vesicle
Describe what a polyprotic acid is and give an example
Any amino acid is an example
What is the Michaelis- Menten equation?
V= Kcat [Enzyme] [S] / (Km + [S])
Where does the protein rotate? What inhibits certain areas from rotating?
It can rotate around the alpha C and C bond as well as the alpha C and N bond. It cannot rotate around the peptide bond because of the partial double bond.
Give 3 facts about negative delta G
-free energy is released
-spontaneous in the forward direction
-favorable
-when a process has +deltaH and -deltaS, it is most likely favorable
Name 3 differences between triglyceride and glycerophospholipid
Triglyceride:
-non polar (no polar region), 3 fatty acid tails, no variable head group, no phosphate group
Glycerophospholipid:
-amphipathic, 2 fatty acid tails, variable head group, phosphate group
**any 3
What % of Glu will have the R group protonated at pH=7.4?
nearly 99%
What is 1 of the assumptions of the Michaelis- Menten kinetics?
1: Initial rate assumption: At initial phase where V depends only on [Et], little to no product, rare reaction, reaction only goes 1 way
2: Steady state assumption: initial rate of reaction results in steady state where [ES] is constant
What is another name for gel filtration? Explain what this technique involves (be very specific)
Size exclusion chromatography
-separates according to size, larger proteins travel faster and elute earlier because they cannot fit through the pores (have to go around), has high capacity, undenatured proteins
**must say at least 3 things about the process
Difference between ∆G and ΔG0'
ΔG: can change, not at standard conditions
ΔG0': cannot change, free energy at biochemical standards
-temp: 25C, pH=7, initial concentration of products+reactants= 1M, [H+]= 10^-7, [H2O]=55.5M
What type of protein does a detergent disrupt and how?
Integral (transmembrane) protein
Detergents have a polar and nonpolar region which allows it to interact with the hydrophobic tail of a protein. This then disrupts the bond and separates them.
Yes, almost every biological process is dependent on pH even if H+ is not directly involved. Many proteins have ionized groups with distinct pKa values which is determined by pH of the environment.