Bond Polarity
Chemical Reactions
Classify the type of element that is at the beginning of the name of every ionic compound, with the sole exceptions being those that have positive polyatomic ions.
What is a metal or the cation atom?
A compound has more than one ________.
What is "element?"
OR what is "type of atom?"
These are the pages of the Chemistry Reference Tables where you can find an element's electronegativity.
What are pages 10 & 11? (Table S)
True or False: A chemical reaction requires a chemical change, aka. a new substance being made.
What is True?
True or False: Something that goes from solid form to aqueous form has undergone a chemical change.
What is False?
[That is a physical change.]
The type of element located on the "staircase" of the Periodic Table.
What is a "metalloid"?
True or False: You must use prefixes when naming ionic compounds.
What is False?
This type of bonding involves a sharing of electrons, in order for each atom to reach stability.
What is covalent bonding?
Other than lowercase deltas with positive/negative symbols, this is the way to draw a depiction of a dipole.
What is a cross-base arrow?
This type of reaction has oxygen gas as a reactant.
What is combustion?
Pairs of electrons shared between the nitrogen atoms in a molecule of N2?
What is "3'?
Of the following elements, helium, hydrogen, phosphorous, potassium, _______________ tends not to react with other elements.
What is, "helium"?
This is the name used to describe a negative ion.
What is an anion?
This type of bonding happens when there is an electron transfer.
What is ionic bonding?
This is the accepted range for the electronegativity difference of a polar bond.
What is 0.4-1.8?
[If you're within 0.1 on either side, you still get the point.]
This is the opposite of synthesis (aka. combination).
What is decomposition?
The difference between a molceule and a crystal?
What is, "the molecule is made of nonmetals only, and the crystal is metal and nonmetal"
or "Molecular compounds engage in covalent bonding, and crystals engage in ionic bonding."
Strontium and barium have similar chemical properties because atoms of these elements have the same number of...
What is "valence electrons"?
This is the formula unit for lithium sulfide.
What is Li2S?
True or False: All atoms are trying to reach the stable octet.
What is False?
(Ex: duet rule with the elements Helium and Hydrogen)
If the EN difference in a bond is classified as less than 0.4 (usually, it's a value of 0), then this is what the bond can be classified as.
What is a nonpolar bond?
OR what is a nonpolar covalent bond?
This type of reaction requires an element & (separate from that element) a compound on the left side of the arrow, as well as a full reactionary exchange between the lone element and one of the elements in the compound.
What is single replacement?
The molecular formula for ammonia is NH3(g), is a compound that is used to manufacture fertilizer for agriculture. This is the number of electrons shared between the nitrogen atom and one of the hydrogen atoms in an ammonia molecule.
What is 2 electrons?
Elements that have atoms with stable valence electron configurations in the ground state are found in...
What is "Group 18"?
The noble gas that has atoms with the same number of electrons as a chloride ion in table salt.
What is "argon"?
If the electronegativity difference in a bond is over 1.8, this is what the bond can be classified as.
What is ionic?
This is a major example of double replacement, often used to help clean and purify things.
What is neutralization?
This is the number of electrons shared in the bond between a hydrogen atom and a carbon atom in the molecule below.
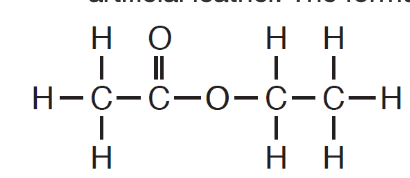
What is two electrons?
On the modern Periodic Table, the elements are arranged in order of increasing...
What is "Atomic number"?
True or False: This correct name for Mg(OH)₂ is magnesium (II) hydroxide.
What is False?
If all identical bonds on a central atom of a molecule appear to have dipoles that cancel each other out, and there are no lone pairs on the central atom, this is the designation given to that molecule.
What is nonpolar?
This is the correct Lewis electron-dot diagram represents a molecule of H2S?
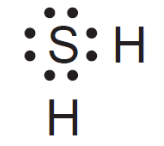
This type of bonding adopts the electron sea model, in which all the metal atoms in a metallic solid contribute their valence electrons to form a “sea” of mobile electrons.
What is metallic bonding?
[No, this will not be on the test, but it could be on the Regents. This is literally all you need to know about metallic bonding for the Regents.]
This is the formula for carbon diselenide.
What is CSe2?
This is the name of a class of ion that is made of multiple atoms, bonded covalently, that operates like a single unit in an ionic bond.
What is a polyatomic ion?
OR what are polyatomic ions?