What is the difference in electrons between ionic and covalent bonds?
Ionic bonds = electrons are transferred
Covalent bonds = electrons are shared
Rule that states that atoms of main-group elements combine so that each aotm has 8 valence electrons.
The octet rule
How many dots would be drawn in the Lewis dot structure for fluorine?
7
Type of compound with high melting points
Ionic or Metallic compounds
How many electrons does hydrogen want total in a covalent bond?
2
Types of elements usually involved in ionic bonding
metal + nonmetal
Structure that shows only the valence electrons in an atom
Lewis dot structure
What Group number has a Lewis dot structure like ?
3 or 13
Type of compound with low melting points
covalent compounds
How old is Mrs. Mortl turning on Sunday
26
Types of element involved in covalent bonding
nonmetals
Draw the lewis structure for H2O

How many dots could be drawn in a Lewis dot structure for an element that can form 3 chemical bonds?
3 or 5
type of compound that is malleable
metallic compound
How many days of school are there until finals? (not counting today)
39
How many total bonds can Nitrogen form?
3 bonds
Draw the Lewis structure for N2
What is the maximum number of chemical bonds that could be formed by an element in Group 6?
2 bonds
ionic compounds will conduct electricity when________
they are dissolved in water/ Melted
Where do you find extra copies of all of the worksheets in class
The numbered folders on the front table!!!!!
A chemical bond that involves the sharing of electrons pairs between atoms
Covalent bond
Draw the Lewis Structure for CO2
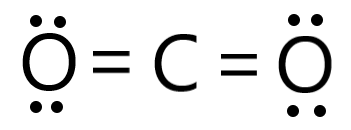
What is the Lewis dot structure for an element that can form only 1 chemical bond?
Covalent compounds are generally in a _______ state at room temperature.
liquid or gas
What is Mrs. Mortl's favorite color?
green :-)