The main plant hormone that helps for growth?
AUXIN

Give definitions for dynamic and static work of muscles?(Main difference)
Dynamic-more actively
Static-contraction for a long time(without rest)
What is the oxidation number of chlorine in
hydrogen chloride?
-1
What is the balanced equation for the reaction
between hydrochloric acid and sodium
hydroxide?
A) HCl + NaOH → NaCl + H2
B) HCl + Na2O → NaCl + H2O2
C) HCl + NaOH → NaCl + H2O
D) 2HCl + Na2O → 2NaCl + H2O
C) HCl + NaOH → NaCl + H2O
The odor of rotten egg comes from …
A) SO3
B) SO2
C) H2S
D) H2SO4
Show the color of sulfur
A) Yellow
B) Blue
C) Purple
D) Green
E) Violet
C) H2S
A) Yellow
Mitotic phase has two stages: mitosis and cytokinesis
Write processes of this 2 stages
Mitosis-nuclear division
1)DNA condenses into visible chromosomes
2)THE SPINDLE FIBERS separate sister chromatids during mitosis
Cytokinesis-cytoplasmic division
Write by alleles:
Dominant homozygous trait
Heterozygous trait
Recessive homozygous trait
1)AA,BB
2)Aa,Bb
3)aa,bb
The main difference between phenotype and genotype?
Phenotype-physical or visible characterictics
Genotype-complete set of genes
What is the most reactive halogen?
Fluorine
How many grams of nitric acid can be produced
from 22.4L of NO2, resulting a following
reaction:
4NO2 + 2H2O + O2 --> 4HNO3
A: 63grams
22.4/22.4=1mole
4____4
1____x. x=1mole
m(HNO3)=M(HNO3)xn(HNO3)=63x1=63grams
One strand of DNA has the base sequence
T A C G A T T G A
What is the complementary strand of DNA?
T A C G A T T G A
A T G C T A A C T
Give 3 main differences between mitosis and meiosis processes?
1)Mitosis-purpose:growth and repair,
Meiosis-purpose:reproduction
2)Mitosis-from diploid two diploid cells
Meiosis-from diploid four haploid cells
3)Mitosis-Result-2 identical daughter cells
Meiosis-Result-4 different daughter cells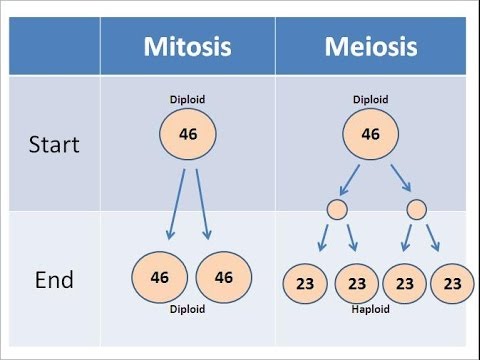
Name 3 main structures of nucleotide

What is the balanced equation for the reaction
between sulfur and oxygen to form sulfur
dioxide?
S + O2 → SO2
A reaction was performed with a theoretical
yield of 15 grams, but only 12 grams of product
was produced. What is the percent yield of this
reaction?
A) 75%
B) 80%
C) 85%
D) 90%
B)80%
actual mass/theoretical mass x100%=12/15x100%=80%
Write main processes for intephse subphases-
1)G1 phase
2)S phase
3)G2 phase
1)G1 phase
•cell grows larger
•many proteins synthesized
•synthesis of organelles
2)S phase
•replication of DNA
•DNA copy itself
•the longest stage
3)G2 phase
•cell continues to grow
•make centrioles
•they have spindle fibers(separating chromosomes)
Show by cross (example with genes)
1) Monohybrid cross
2) Dihybrid cross
1)Monohybrid cross-only one trait
Ex-AA х аа
2)Dihybrid cross-two traits
Ex-AABB х aabb

Which of the following is NOT an oxidation
state of sulfur?
A) +4
B) +6
C) -2
D) 0
E) -4
E) -4
What will be the color of litmus paper dipped in
HCl solution?
A) Red
B) Blue
C) Violet
D) Green
E) Black
A) Red
How many valence electrons are there in an
atom of chlorine?
A) 5
B) 6
C) 7
D) 8
E) 2
C) 7
What will be the result in F1 generation when a homozygous white male rabbit is crossed with a homozygous black female rabbit?

In a dihybrid cross, SsYy x SsYy, what fraction of the offspring will be homozygous for both traits?

 4/16
4/16
Which one is NOT correct for H2SO4?
A) It is colorless, nonvolatile liquid
B) Its one of the most important chemical
compounds
C) It is a strong acid
D) Its aqueous solutions are good conductors
of electricity
E) It does not react with alkali metals
E) It does not react with alkali metals
6 g of hydrogen reacts completely according to
the following reaction:
N2 + 3H2 → 2NH3
If 17 g of NH3 is produced, what is the percent
yield for this reaction?
(N=14g/mol, H=1g/mol)
A) 10%
B) 25%
C) 50%
D) 75%
E) 100%
C) 50%
6/2=3mole.
3____2
3____x x=2mole
2x17=34g
17/34=?% =50%
For the balanced equation shown below, if the
reaction of 252 grams of C6H6O3 produces a
25.0% yield, how many grams of H2O would be
produced ?
(C=12g/mol, H=1g/mol, O=16g/mol)
C6H6O3+6O2=>6CO2+3H2O
A) 9
B) 18
C) 27
D) 54
E) 108
C 27